From Bioethics Briefings
Stem Cells
Highlights
- Stem cell research is proceeding rapidly around the world.
- Stem cells hold great promise for treating degenerative conditions such as Parkinson’s disease and diabetes, understanding genetic illnesses, and answering fundamental questions about human development.
- The main ethical objections are to human embryonic stem cell research, because early-stage human embryos are destroyed during the process of deriving their stem cells.
- Induced pluripotent stem (iPS) cells, created by reprogramming human somatic cells (cells other than eggs, sperm, or embryos), represents a new kind of stem cell research which doesn’t involve use of human embryos.
- IPS cells are unlikely to eliminate the need for human embryonic stem cells in research for many reasons.
- Scientific standards for preclinical testing and clinical trials involving stem cell-based therapies are urgently needed.
- Stem cell tourism poses a new ethical challenge to the field since it is difficult to regulate and because it exploits patients who are desperate for new treatments.
Framing the Issue
Stem cells are undifferentiated cells that have the capacity to renew themselves and to specialize into various cell types, such as blood, muscle, and nerve cells. Embryonic stem cells, derived from five-day-old embryos, eventually give rise to all the different cells and organ systems of the embryo. Embryonic stem cells are pluripotent, because they are capable of differentiating along each of the three germ layers of cells in the embryo, as well as producing the germ line (sperm and eggs). The three germ layers are the ectoderm (skin, nerves, brain), the mesoderm (bone, muscle), and the endoderm (lungs, digestive system).
During later stages of human development, minute quantities of more mature stem cells can be found in most tissue and organ systems, such as bone marrow, the skin, and the gut. These are somatic stem cells, responsible for renewing and repairing the body’s specialized cells. Although the lay public often refers to them as “adult” stem cells, researchers prefer to call them multipotent because they are less versatile than pluripotent stem cells, and because they are present from the fetal stage of development and beyond. Multipotent stem cells can only differentiate into cells related to the tissue or organ systems from which they originated – for instance, multipotent blood stem cells in bone marrow can develop into different types of blood cells, but not into nerve cells or heart cells.
While multipotent stem cell research has been around for nearly 50 years and has led to clinical therapies for leukemia and other blood disorders, the field of human embryonic stem cell research is still relatively new, and basic discoveries have yet to be directly transitioned into clinical treatments. Human embryonic stem cells were first isolated and maintained in culture in 1998 by James Thomson and colleagues at the University of Wisconsin. Since then, more than a thousand different isolates–“lines” of self-renewing embryonic stem cells–have been created and shared by researchers worldwide.
The main ethical and policy issues with stem cells concern the derivation and use of embryonic stem cells for research. A vocal minority of Americans objects to the destruction of embryos that occurs when stem cells are derived. Embryonic stem cell research is especially controversial for those who believe that five-day-old preimplantation human embryos should not be destroyed no matter how valuable the research may be for society.
To bypass this ethical controversy, the President’s Council on Bioethics recommended in 2005 that “alternative sources” of pluripotent stem cells be pursued. Some alternatives have been developed, most notably, the induced pluripotent stem (iPS) cells – human skin cells and other body cells reprogrammed to behave like embryonic cells. But embryonic stem cell research will remain needed because there are some questions only they have the potential to answer.
Disease-in-a-Dish: The Promise of Embryonic Stem Cells
Embryonic stem cells are necessary for several aims of scientific and biomedical research. They include addressing fundamental questions in developmental biology, such as how primitive cells differentiate into more specialized cells and how different organ systems first come into being. By increasing our knowledge of human development, embryonic stem cells may also help us better understand the causes of fetal deformations.
Other important applications lie in the areas of disease research and targeted drug development. By deriving and studying embryonic or other pluripotent stem cells that are genetically-matched to diseases such as Parkinson’s disease and juvenile diabetes, researchers are able to map out the developmental course of complex medical conditions to understand how, when, and why diseased specialized cells fail to function properly in patients. Such “disease-in-a-dish” model systems provide researchers with a powerful new way to study genetic diseases. Furthermore, researchers can aggressively test the safety and efficacy of new, targeted drug interventions on tissue cultures of living human cells derived from disease-specific embryonic stem cells. This method of testing can reduce the risks associated with human subjects research.
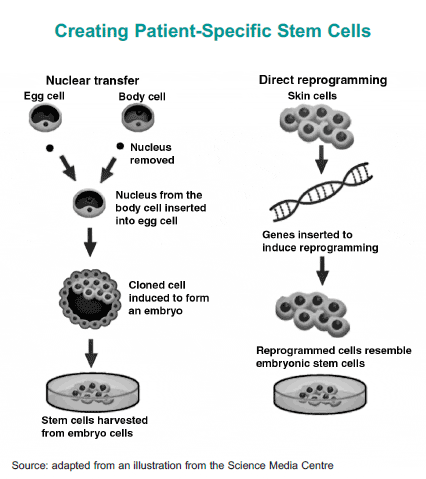
One possible way of deriving disease-specific stem cells is through a technique called somatic cell nuclear transfer (SCNT), otherwise known as “research cloning.” By replacing the DNA of an unfertilized egg with the DNA of a cell from a patient’s body, researchers are able to produce embryonic stem cells that are genetically-matched to the patient and his or her particular disease. SCNT, however, is technically challenging and requires the collection of high-quality human eggs from female research volunteers, who must be asked to undergo physically burdensome procedures to extract eggs.
A much more widespread and simpler technique for creating disease-specific stem cells was pioneered in 2006 by Shinya Yamanaka and colleagues in Kyoto, Japan. They took mouse skin cells and used retroviruses to insert four genes into them to to create iPS cells. In 2007, teams led by Yamanaka, James Thomson, and George Daley each used similar techniques to create human iPS cells. The iPS cell approach is promising because disease-specific stem cells could be created using skin or blood samples from patients and because, unlike SCNT, it does not require the procurement of human eggs for research.
However, despite these advances, scientists do not believe iPS cells can replace human embryonic stem cells in research. For one, embryonic stem cells must be used as controls to assess the behavior and full scientific potential of iPS cells. Furthermore, iPS cells may not be able to answer some important questions about early human development. And safety is a major issue for iPS cell research aimed at clinical applications, since the cell reprogramming process can cause harmful mutations in the stem cells, increasing the risk of cancer. In light of these and other concerns, iPS cells may perhaps prove to be most useful in their potential to expand our overall understanding of stem cell biology, the net effect of which will provide the best hope of discovering new therapies for patients.
Ethical and Policy Issues
Many who oppose embryonic stem cell research believe for religious or other personal reasons that all preimplantation embryos have a moral standing equal to living persons. On the other hand, those who support embryonic stem cell research point out that not all religious traditions grant full moral standing to early-stage human embryos.
According to Jewish, Islamic, Hindu, and Buddhist traditions, as well as many Western Christian views, moral standing arrives much later during the gestation process, with some views maintaining that the fetus must first reach a stage of viability where it would be capable of living outside the womb. Living in a pluralistic society such as ours, supporters argue, means having to tolerate differences in religious and personal convictions over such theoretical matters as when, during development, moral standing first appears.
Other critics of embryonic stem cell research believe that all preimplantation embryos have the potential to become full-fledged human beings and that they should never have this potential destroyed. In response, stem cell supporters argue that it is simply false that all early-stage embryos have the potential for complete human life – many fertility clinic embryos are of poor quality and therefore not capable of producing a pregnancy (although they may yield stem cells). Similarly, as many as 75% to 80% of all embryos created through intercourse fail to implant. Furthermore, no embryos have the potential for full human life until they are implanted in a woman’s uterus, and until this essential step is taken an embryo’s potential exists only in the most abstract and hypothetical sense.
Despite the controversies, embryonic stem cell research continues to proceed rapidly around the world, with strong public funding in many countries. In the U.S., federal money for embryonic stem cell research is available only for stem cell lines that are on the National Institutes of Health stem cell registry. However, no federal funds may be used to derive human embryonic stem cell lines; NIH funds may only be used to study embryonic stem cells that were derived using other funding sources.
Despite the lack of full federal commitment to funding embryonic stem cell research in the U.S., there are wide-ranging national regulatory standards. The National Academy of Sciences established guidelines in 2005 for the conduct of human embryonic stem cell research. (See Resources.) According to these guidelines, all privately and publicly funded scientists working with embryonic stem cells should have their research proposals approved by local embryonic stem cell research oversight (ESCRO) committees. ESCRO committees are to include basic scientists, physicians, ethicists, legal experts, and community members to look at stem-cell-specific issues relating to the proposed research. These committees are also to work with local ethics review boards to ensure that the donors of embryos and other human materials are treated fairly and have given their voluntary informed consent to stem cell research teams. Although these guidelines are voluntarily, universities and other research centers have widely accepted them.
At the global level, in 2016 the International Society for Stem Cell Research (ISSCR) released a comprehensive set of professional guidelines for human stem cell research, spanning both bench and clinical stem cell research. (See Resources.) Unlike the NAS guidelines, the ISSCR guidelines go beyond American standards, adding, for example, the recommendation that stem cell lines be banked and freely distributed to researchers around the world to facilitate the field’s progress on just and reasonable terms.The potential for over-commercialization and restrictive patenting practices is a major problem facing the stem cell field today, which may delay or reduce the broad public benefit of stem cell research. The promise of broad public benefit is one of the justifying conditions for conducting stem cell research; without the real and substantial possibility for public benefit, stem cell research loses one of its most important moral foundations.
However, providing useful stem-cell-based therapies in the future is not a simple proposition, either. Developing a roadmap to bring stem cell research into the clinic will involve many complex steps, which the new ISSCR guidelines help address. They include:
- Uniform standards for cell processing and manufacturing.
- Preclinical testing requirements using animal models before first-in-human clinical trials can begin with pluripotent stem-cell-based biological products
- Fair and appropriate procedures for enrolling human subjects in early clinical trials
- Standards for assessing risk-benefit ratios and the use of placebo controls.
These and other difficult issues must be sorted out if stem cell research in all its forms is to fulfill its promise.
STEM CELL GLOSSARY
- Pluripotent – embryonic stem cells; capable of differentiating into all cell types
- Multipotent – cells other than embryonic cells that are capable of differentiating into a limited variety of cells related to a particular tissue system
- Somatic cell nuclear transfer – research cloning; replacing the DNA of an unfertilized egg with the DNA of a cell from a patient
- Induced pluripotent stem (iPS) cells – multipotent stem cells that are reprogrammed to behave like embryonic stem cells
Latest Ethical Concerns
Newer ethical issues in stem cell research go far beyond the embryo debate, since they encompass all stem cell types, not just human embryonic stem cells, and because they involve human subjects who, despite what one may think about the moral status of preimplantation embryos, are unequivocally moral persons. No other emerging issue better encapsulates the above concern than the growing phenomenon of stem cell tourism. At present, stem cell-based therapies are the clinical standard of care for only a few conditions, such as hematopoietic stem cell transplants for leukemia and epithelial stem cell-based treatments for burns and corneal disorders. Unfortunately, some unscrupulous clinicians around the world are exploiting patients’ hopes by purporting to provide – for large sums of money – effective stem cell therapies for many other conditions. These so-called “stem cell clinics” advance claims about their proffered stem cell therapies without credible scientific rationale, transparency, oversight, or patient protections.
The administration of unproven stem cell interventions outside of carefully regulated research protocols endangers patients and jeopardizes the legitimate progress of translational stem cell scientific research. Patients who travel for unproven stem cell therapies put themselves at risk of physical and financial harm.
The ISSCR guidelines are a good point for thinking about this important problem. The guidelines allow for exceptional circumstances in which clinicians might attempt medically innovative care in a very small number of seriously ill patients, subject to stringent oversight criteria. These criteria include: independent peer review of the proposed innovative procedure and its scientific rationale; institutional accountability; rigorous informed consent and close patient monitoring; transparency; timely adverse event reporting; and a commitment by clinician-scientists to move to a formal clinical trial in a timely manner after experience with at most a few patients. By juxtaposing some current stem cell clinics against the standards outlined in the ISSCR guidelines, one may easily identify some clinics’ shortcomings and call into question the legitimacy of their purported claims of providing “innovative care” to patients.
Moving beyond past debates about embryo status to issues concerning the uses of all varieties of stem cells, one can begin to focus the bioethical discourse on areas that have a much broader consensus base of shared values, such as patient and research subject protections and justice. Justice may also call on regulatory and oversight bodies to include a greater involvement of community and patient advocates in the oversight of research. Dealing with the bioethics of stem cell research demands that we wrestle with these and other tough questions.
Insoo Hyun, PhD, is the director of the Center for Life Sciences and Public Learning at the Museum of Science, Boston.
45% of our work is supported by individual donors like you. Support our work.
Resources
- International Society for Stem Cell Research (ISSCR), Guidelines for Stem Cell Research and Clinical Translation (2016). The ISSCR is an independent, nonprofit organization which promotes and fosters the exchange and dissemination of information and ideas relating to stem cells, to encourage the general field of research involving stem cells and to promote professional and public education in all areas of stem cell research and application.
- National Academies, Final Report of the National Academies' Human Embryonic Stem Cell Research Advisory Committee and 2010 Amendments to the National Academies' Guidelines for Human Embryonic Stem Cell Research (National Academies Press, 2010).
- Jonathan Kimmelman, et al., “Global Standards for Stem-Cell Research,” Nature 533, no. 7603 (2016): 311-313.
- Insoo Hyun, Bioethics and the Future of Stem Cell Research (Cambridge University Press, 2013).
- Insoo Hyun, Konrad Hochedlinger, Rudolf Jaenisch, and Shinya Yamanaka, “New Advances in iPS Cell Research Do Not Obviate the Need for Human Embryonic Stem Cells,” Cell Stem Cell 1, no. 4 (2007): 367-68.
- Dina Gould Halme and David A. Kessler, “FDA Regulation of Stem-Cell-Based Therapies,” The New England Journal of Medicine 355, no. 16 (2006): 1730-35.
Experts
- George Q. Daley, MD, PhD
- M. William Lensch, PhD
- Insoo Hyun, PhD
- Josephine Johnston, LLB, MBHL
- Bernard Lo, MD