The Hastings Center Bioethics Timeline
“Bioethics” has been defined in several different ways. Most broadly, it is the interdisciplinary study of ethical, legal, and social issues arising in the life sciences and health care. Though it has roots tracing back decades or generations earlier, modern bioethics is widely held to have arisen in the late 1960s, roughly around the time of the founding of The Hastings Center in 1969. Since then, it has helped transform the practice of medicine and inform policy-making about myriad issues concerning the life sciences, from public health and delivery of medical care to agricultural biotechnology.
In commemoration of The Hastings Center’s 50th anniversary, a committee of scholars drawn from The Hastings Center’s fellows came together to develop a timeline of the history of bioethics. The product of that effort is of course limited in many ways: it can accommodate only a few of the many items that might have been included; it forefronts the history of bioethics in the United States; it emphasizes ethical issues involving humans more than those involving nonhuman organisms; it depends on the committee’s collective (and inherently subjective) judgments about historical significance; and it identifies and describes a set of salient moments in bioethics rather than offering a unified narrative of the field’s development. In addition, there were a number of pragmatic challenges in creating the timeline. For instance, the categories we used to “lump” items into thematic groups required some arbitrary decisions, as the field of bioethics contains a wide array of sub-fields, and sub-sub-fields. In the end, to keep the list of themes to a reasonable number, we undoubtedly put items in the same category that others might argue deserve to be put in their own domains.
Another limitation is that the timeline begins in 1946 after the conclusion of World War II. The decision to have the timeline begin at this moment anchors it to the importance of the Nuremberg Doctors’ Trial in the history of bioethics in the United States. In this tribunal, high-ranking Nazi doctors and administrators were tried for war crimes; crimes against humanity; and medical experiments without consent, on prisoners of war, civilians of occupied countries, and others. Their crimes included murder (including mass murder), brutality, torture, and other inhuman acts.
In deciding what to include in the timeline, books posed a particularly challenging problem, as there are far too many significant books in the field to list them all. The committee decided to solicit “top-10 books in bioethics” recommendations from 14 senior national and international bioethicists, and then supplemented these suggestions with a similar survey sent to all Hastings Center fellows. Books that were recommended by senior bioethicists and members of the bioethics timeline committee, as well as those that received two or more nominations from the larger group of fellows, were embedded into the timeline. All recommended books are also listed by the year of publication in the Appendix.
The timeline’s limitations notwithstanding, it is a useful starting point in understanding bioethics—how and when it emerged, the issues it has taken on, the arc of thinking within it, and some of its major contributors.
The timeline has benefitted from input from many scholars besides those on the committee. Many suggested items for inclusion, and some also reviewed and commented on the timeline as it was developed. It is unavoidably no more than a work in progress, however, and the committee welcomes suggestions for later iterations. To make a suggestion, send an email to communications@thehastingscenter.org.
We hope that the timeline will not merely serve as a static historical exercise. Perhaps it can serve as a catalyst for reflecting on the development of the field of bioethics. What has been accomplished? Where should the field go next?
The timeline is dedicated to Daniel Callahan and Willard Gaylin, without whom there would have been no Hastings Center.
The Hastings Center Bioethics Timeline Committee
–Robert A. Pearlman, MD, MPH (Chair)
–Robert Baker, PhD
–Marion Danis, MD
–Arthur R. Derse, MD, JD
–Gregory E. Kaebnick, PhD
–Susan E. Lederer, PhD
–Barron H. Lerner, MD, PhD
–Kathleen E. Powderly, CNM, PhD (d. 2021)
–Matthew K. Wynia, MD, MPH (Vice Chair)
1946
Ethics of Health Policy
Hill-Burton Act
Congress enacted the Hospital Survey and Construction Act (Hill-Burton Act), which provides for hospitals, nursing homes, and other health facilities grants and loans for construction and modernization. Intended to insure 4.5 hospital beds per 1,000 people, the Act made hospital care more readily available in many parts of the United States.
Research Ethics
Guatemala Syphilis Studies
The Syphilis Study Section of the National Institutes of Health-approved grants for Public Health Service researchers to study intentional infection with syphilis, gonorrhea, and chancroid, followed by treatment with newly available penicillin. The studies, involving more than 1,300 Guatemalan soldiers, prisoners, and mental patients, began in 1946 and ended in 1948. The studies remained buried in the archives until 2010. See timeline 2011.
Research Ethics
AMA Adopts Principles for Permissible Human Experimentation
The Judicial Council of the American Medical Association (AMA), at the behest of researcher Andrew C. Ivy, approved three requirements for human subjects research: voluntary consent of the subject, prior animal experimentation to determine risk, and proper medical management of the experiment. These requirements were intended to buttress claims about conventions of experiments involving humans in light of the Nuremberg Doctors’ Trial
Research Ethics
United States v. Karl Brandt et al.

An American military tribunal presides at the trial of 23 high ranking Nazi doctors and administrators. These individuals were charged with war crimes, crimes against humanity, and medical experiments, without consent, on prisoners of war, civilians of occupied countries, and others, which included murder, brutality, torture, atrocities, and other inhuman acts.
Annas, G. J., “Beyond Nazi War Crimes Experiments: The Voluntary Consent Requirement of the Nuremberg Code at 70,” American Journal of Public Health 108, no. 1 (2018): 42-46.
1947
Research Ethics
Nuremberg Code
The judges in the Nuremberg Doctors Trial issue requirements for “Permissible Medical Experiments” with which to judge the Nazi doctors. The document outlines ten principles. The first and most widely cited principle states that “the voluntary consent of the human subject is absolutely essential.” The document, which has come to be called the Nuremberg Code, has been hailed as the most important document in the history of the ethics of medical research.
Health & Human Services, Office for Human Research Protection
1948
Professionalism and Ethics
The Declaration of Geneva

Established in 1947 when physicians from 27 nations met “to work for the highest possible standards of ethical behavior and care by physicians,” the World Medical Association adopted the Declaration of Geneva as a new secular physician’s oath in the aftermath of the Second World War. The Declaration of Geneva modifies language from versions of the Hippocratic Oath, and includes the provision that the physician must “not use medical knowledge contrary to the laws of humanity.” The Declaration was adopted shortly before the General Assembly of the United Nations adopted the Universal Declaration of Human Rights.
International Ethics
Universal Declaration of Human Rights
The United Nations, founded in 1946, issued the Universal Declaration of Human Rights. Composed by a committee headed by Eleanor Roosevelt, former first lady of the United States, the Declaration sets out rights to which all human beings are entitled and became a foundational document for international bioethics.
International Ethics
The World Health Organization
The World Health Organization was created in 1948 to focus on health affairs within the newly established United Nations. The WHO adopted an expanded definition of health: “Health is a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity.” Initially the WHO prioritized control of malaria, tuberculosis, venereal disease, and other communicable diseases, plus women and children’s health, nutrition, and sanitation. The WHO has organized Collaborating Centers for Bioethics to further its ethics goals
1949
International Bioethics
International Code of Medical Ethics
In 1949, the third Assembly of the World Medical Association enacted an International Code of Medical Ethics, the first effort at globalizing medical ethics and the responsibilities of doctors and patients.
1952
Sexual and Gender Ethics
Sex Reassignment Surgery
Christine Jorgensen became the first American widely known for undergoing sex reassignment surgery. Born George William, Jorgensen served in the military during World War II. After undergoing several surgeries in Denmark, Jorgensen returned to the United States and underwent the new procedure of vaginoplasty. Endocrinologist Harry Benjamin credited Jorgenson with advancing his research in transgenderism. Jorgensen became a highly visible advocate for the transgender community.
Meyerowitz, J., How Sex Changed (Harvard University Press, 2009).
End-of-Life Issues
Technology
Clinician-Patient Relationship
Artificial Ventilator
Responding to a polio epidemic in Copenhagen, anesthesiologist Bjorn Ibsen, a protégé of Harvard anesthesiologist Henry K. Beecher, developed the first practical artificial ventilator. The device became a core technology underlying the creation of intensive care units (ICUs). Ibsen established the world’s first ICU in Copenhagen the following year. Use of the machine undermined traditional standards of cardio-pulmonary criteria for defining death.
1953
Research Ethics
The Wilson Memorandum
Secretary of Defense Charles E. Wilson sends a Memorandum to the Secretaries of Army, Navy, and Air Force on the “Use of Human Volunteers in Experimental Research.” A slightly revised version of the Nuremberg Code, the principles are to be used in medical research involving American military personnel. The document is labeled “top secret” so that access is limited. This illustrates an ongoing discussion among medical researchers about the appropriate conduct of clinical research.
Genomics and Ethics
The Double Helix of DNA

James D. Watson and Francis Crick announce they have determined the double-helical structure of DNA, the molecule containing human genetic material. Aided by the crystallographer Rosalind Franklin and Maurice Wilkins, the discovery marked a significant milestone in the history of biology and helped foster modern molecular biology.
Technology
Religion and Ethics
Papal Approval of Organ Transplantation
At the 26th Congress of Urology, Pope Pius XII, quoting St. Thomas Aquinas (Summa Theologica II, Question 65, Article I) endorsed organ transplants on the grounds of the Totality Principle, which stated that every part of the human body “exists for the sake of the whole.” This permitted the sacrifice of one part of a human being for the continued survival of another.
1954
Ethical Theory and Methods
Publication of Joseph Fletcher’s Morals and Medicine
Fletcher (1905-1991), an ordained Episcopal priest and professor of ethics who wrote 11 books and more than 350 articles, was a seminal figure in modern biomedical ethics. In Morals and Medicine, Fletcher advanced the case for active euthanasia, for truth-telling with dying patients, for artificial insemination, and most radically, for the sexual sterilization of individuals judged to be unfit for parenthood.
J. Fletcher, Morals and Medicine (Princeton: Princeton University Press, 1954).
S. Toulmin, “How Medicine Saved the Life of Ethics,” Perspectives in Biology and Medicine 25, no. 4 (1982): 736-50.
Technology
Successful Kidney Transplants

Dr. John Merrill explains the workings of a then new machine called an artificial kidney to Richard Herrick, left, and his brother Ronald.
In December 1954 surgeon Joseph Murray and his colleagues at the Peter Bent Brigham Hospital in Boston performed the first successful human kidney transplant. During the surgery, 24-year-old Richard Herrick received a kidney donated by his identical twin brother, Ronald. Richard lived for nine years with the repurposed kidney. Kidney (and soon other organ) transplants stimulated much ethical discussion and debate over risks to living donors, whether mentally impaired individuals could “donate,” a market in human organs, and how to allot scarce tissue.
J. E. Murray, “Reflections on the First Successful Kidney Transplantation,” World Journal of Surgery 6, no. 3 (1982): 372-76.
R. M. Veatch, and L. F. Ross, Transplantation Ethics (Georgetown University Press, 2015).
Social Justice
Brown v. Board of Education, Topeka, Kansas
In this landmark decision of the U.S. Supreme Court, the Court ruled unanimously (9-0) that state laws upholding racial segregation in public schools were unconstitutional. Undoing the doctrine of “separate but equal” established by the Supreme Court in Plessy v. Ferguson (1896), the Justices ruled “separate educational facilities are inherently unequal,” and violated the Equal Protection Clause of the 14th Amendment to the U.S. Constitution. The decision helped spur the Civil Rights movement.
1955
Technology
Research Ethics
Jonas Salk and the Polio Vaccine
In 1954, Jonas Salk (1914-1995), with funding from the National Foundation for Infantile Paralysis, launched a massive clinical trial of his new vaccine against polio. Before this trial in which more than 1.8 million American children between the ages of six and nine participated, Salk tested the vaccine on institutionalized, mentally incapacitated children. Parents of the “Polio Pioneers” signed a form “requesting that their child participate” in the trial. On April 12, 1955, Thomas Francis, Salk’s mentor and the director of the trial, reported that the vaccine was safe and 90% effective in protecting against paralytic poliomyelitis. The success of the vaccine prompted extraordinary support for medical science and vaccination among the American public.
D. Oshinsky, Polio: An American Story (Oxford University Press, 2005).
1956
Research Ethics
Hepatitis Experiments at Willowbrook

Dr. Saul Krugman begins experiments at Staten Island’s Willowbrook State School in which developmentally disabled children are given active hepatitis virus in order to study the disease. It lasts until 1970 and becomes one of the most infamous research scandals of the era.
1957
End-of-Life Issues
Religion and Ethics
Pope Pius XII Address on Use of Ventilators
In an address delivered to the International Congress of Anesthesiologists, The Prolongation of Life, Pope Pius XII states, in response to questioning, that it is not obligatory to initiate or to continue artificial ventilation when a patient is expected to inevitably die. He also states that the criteria for death cannot be deduced from any religious and moral principle and does not fall within the competence of the Church. The Pope’s recommendation is subsequently litigated in American law when a Roman Catholic family, the Quinlans, requests that doctors disconnect ventilator support for their daughter, Karen Ann (In re Quinlan [70 N.J. 10, 355 A.2d 647 (NJ 1976)]). This papal allocution is the sole reference in the 1968 Report of the Harvard Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain Death.
Informed Consent
Salgo v. Leland Stanford Jr. University Board of Trustees
A California court first uses the term “informed consent” when ruling for the plaintiff in a malpractice case called Salgo v. Leland Stanford Jr. University Board of Trustees.This concept would become the basic tenet of medical decision-making.
1960
Technology
Inauguration of Chronic Kidney Dialysis
University of Washington nephrologist Belding Scribner develops a shunt that connected the arterial and venous blood systems (later called the “Scribner Shunt”) that enabled doctors to regularly dialyze the blood of patients with kidney failure, thereby saving their lives.
Informed Consent
Natanson v. Kline
In this case, the Kansas Supreme Court built on the 1957 Salgo informed consent concept, further holding the medical profession responsible for the standard of disclosure of risks prior to a procedure.
Natanson v. Kline, 186 Kan. 393, 411, 350 P. 2d 1093.
1961
Ethics of Health Policy
Thomas Szasz Testifies about Mental Illness
Psychiatrist Thomas Szasz testifies in front of the U.S. Congress in a session called “The Constitutional Rights of the Mentally Ill.” He argues that mainstream psychiatry wrongly labels as “diseased” those people who behaved in ways that society finds unusual or disturbing. Szasz would become a major proponent of the “myth” of mental illness and an advocate for deinstitutionalization of the mentally ill.
Research Ethics
Social Responsibility in Pediatric Research
Conference on “Social Responsibility in Pediatric Research” held by the Law-Medicine Research Institute at Boston University. This meeting was one of several meetings, organized by the LMRI and funded by the National Institutes of Health, to investigate clinical research practices.
Research Ethics
Social Justice
The Trial of Adolf Eichmann

An Israeli Court tries and convicts Nazi official Adolf Eichmann of crimes against humanity, war crimes, and crimes against the Jewish people. Among the issues again brought before the public were the inhumane experiments performed by Nazi physicians.
Research Ethics
Thalidomide
Francis Kathleen Oldham Kelsey, a reviewer for the U.S. Food and Drug Administration refuses to approve an application filed in 1960 by William S. Merrill Company to market a German drug, thalidomide, in the United States citing a letter in the British Medical Journal reporting cases of peripheral neuritis—nerve damage in the hands and feet—among patients treated with thalidomide. “The burden of proof that the drug is safe . . . lies with the applicant,” Kelsey wrote to the company on May 5, 1961.
1962
Research Ethics
Publication of Maurice Pappworth’s Human Guinea Pigs: A Warning
British physician Maurice Pappworth publishes an article in the literary magazine Twentieth Century on human experimentation. He raised similar issues about lack of informed consent that would later be raised by Henry Beecher in the United States. The two “whistleblowers” corresponded with and supported each other’s efforts; both referenced violations of the informed voluntary consent requirement of the Nuremberg Code in their critiques of current research ethics violations.
Ethics of Health Policy
Congress Passes Kefauver-Harris Amendment
The Kefauver-Harris amendment to the Federal Food, Drug and Cosmetic Act, which mandated safety and efficacy testing prior to the marketing of drugs, is passed by Congress. It was written in response to the Washington Post’s front-page expose of the thalidomide tragedy, in which about 8,000 infants with missing or malformed limbs were linked to thalidomide’s widespread usage among pregnant women (mostly in Europe) after having taken the drug for nausea.
Resource Allocation
Technology
Publication of Shana Alexander’s Article on Seattle “God Squad”
Life magazine publishes Shana Alexander’s article, “They Decide Who Lives, Who Dies.” It detailed the complicated ethical deliberations of the Admissions and Policies Committee of the Seattle Artificial Kidney Center, which had been established to allocate the use of limited hemodialysis machines in the wake of Belding Scribner’s shunt (see 1960). This story is a fascinating chapter in the history of rationing.
Arts and Ethics
Publication of Ken Kesey’s One Flew Over the Cuckoo’s Nest

Ken Kesey publishes the novel One Flew Over the Cuckoo’s Nest, a scathing rebuke of psychiatry, including its use of forced hospitalization and procedures such as lobotomy.
Kesey, K., One Flew Over the Cuckoo’s Nest (New York: Viking Press, 1962).
Environmental Ethics
Publication of Rachel Carson’s Silent Spring
The New Yorker publishes Rachel Carson’s three-part series, “Silent Spring,” which documented the environmental and health concerns raised by the chemical industry’s indiscriminate use of pesticides. A book of the same name was also published in 1962.
Carson, R., Silent Spring (June 16, 23 and 30 in the New Yorker; book—Cambridge: Houghton Mifflin, 1962).
1963
Resource Allocation
Technology
World’s First Liver Transplant
Dr. Thomas Starzl performs the world’s first liver transplant on Bennie Solis, a 3-year-old boy with biliary atresia. Although Solis and many of the other early recipients of livers die, Starzl and other surgeons eventually perfect the technique and save thousands of lives.
Research Ethics
Stanley Milgram’s Behavior Experiments

Yale University Psychologist Stanley Milgram publishes the first article from his controversial study “Behavioral Study of Obedience” in the Journal of Abnormal and Social Psychology. Milgram’s experimental subjects willingly inflicted apparently painful stimuli to participants because they were told to do so.
Social Justice
Simkins v. Moses H. Cone Memorial Hospital
On November 1, 1963, the Fourth Circuit Court of Appeals in Simkins v. Moses H. Cone Memorial Hospital ruled the separate-but-equal clause of the Hill-Burton Act unconstitutional. Since the U.S. Supreme Court refused to hear the case on appeal, the decision stood. The decision only affected hospitals receiving Hill-Burton funds. A 1964 federal court decision, Eaton v. Grubbs, broadened the prohibitions against racial discrimination to include hospitals that did not receive such funds.
Simkins v. Moses H. Cone Memorial Hospital, 323 F.2d 959 (4th Cir. 1963)
1964
Informed Consent
Application of the President and Dir. of Georgetown College
In Application of the President and Dir. of Georgetown College, the U.S. Circuit Court of Appeals declines to hear a case in which a Jehovah’s Witness, Jesse Jones, argued that she had received a blood transfusion against her will. Eventually, courts would decide that transfusing Jehovah’s Witnesses who object to blood products is unethical.
Application of President & Directors of Georgetown College, Inc., 331 F.2d 1000, 1964 U.S. App. LEXIS 6510, 118 U.S. App. D.C. 80, 9 A.L.R.3d 1367 (D.C. Cir. Feb. 3, 1964).
Research Ethics
Injection of Cancer Cells without Consent
The Jewish Chronic Disease Hospital is accused in Brooklyn Supreme Court of injecting cancer cells into noncancer patients without their consent. This experiment, run by Memorial-Sloan Kettering oncologist Chester Southam, is one of those cited in Henry Beecher’s 1966 exposé of ethically questionable research.
Professionalism and Ethics
Publication of Louis Lasagna’s The Modern Physicians’ Oath
Dr. Louis Lasagna of Tufts University writes an updated version of the Hippocratic Oath. Reflecting concerns of the time, Lasagna’s version cautioned against overtreatment of patients, stressed prevention over cure and contained the controversial idea that at times it was acceptable for physicians to end patients’ lives.
Research Ethics
The Declaration of Helsinki
The World Medical Association issues the Declaration of Helsinki, its recommendations guiding doctors performing clinical research. The Declaration supplemented the Nuremberg Code’s landmark defense of research subjects’ rights, which, by mandating the informed voluntary consent of research subjects, inadvertently precluded research on children and others incapable of consenting. The Declaration clarified these issues for researchers permitting surrogate consent and similar accommodations to the realities of clinical research.
Ethics of Health Policy
Surgeon General’s Report on Smoking
Surgeon General Luther Terry issues Smoking and Health, a report using epidemiological studies to demonstrate that cigarette smoking caused lung cancer and likely heart disease. This was a landmark study in both the history of public health and health activism.
Social Justice
Civil Rights Act

President Lyndon Johnson signs the Civil Rights Act, legally ending discrimination in public places based on race, color, religion, sex, and national origin under any program or activity receiving federal financial assistance.
Social Justice
Medical Committee for Human Rights
A group of health professionals founds the Medical Committee for Human Rights, which provided medical care to individuals fighting for human rights during the Mississippi “Freedom Summer.”
1965
Ethics of Health Policy
Reproductive Ethics
Griswold v. Connecticut
In Griswold v. Connecticut, the U.S. Supreme Court ruled that a Connecticut law prohibiting the use of birth control violated the right of marital privacy—which is within the penumbras of specific guarantees of the Bill of Rights. The ruling legalized contraception for married people.
Griswold v. Connecticut, 381 U.S. 479 (1965)
Research Ethics
Letter Questioning Tuskegee Study
Detroit physician Irwin Schatz writes to the U.S. Public Health Service questioning the morals of the Tuskegee Study of Untreated Syphilis in the Negro Male, but his letter is ignored. Seven years later, there would be widespread outrage when details of the study went public.
Ethics of Health Policy
Congress Passes Medicaid and Medicare
Despite opposition from the American Medical Association, two governmental health insurance programs are passed: Medicaid, which insures the indigent, and Medicare, which insures those 65 and older. The implementation of Medicare, along with the 1963 Fourth Circuit Court of Appeals case mandating desegregation of hospitals and linking desegregation with federal funding policies, transformed hospitals from among the most segregated to the most integrated institutions in the nation. The National Medical Association, which represents the interests of African American patients and physicians, supported both Medicaid and Medicare.
1966
Research Ethics
Publication of Henry Beecher’s Ethics and Clinical Research
Henry K. Beecher, a Harvard anesthesiologist, publishes a seminal article on research involving human subjects in the New England Journal of Medicine. This article describes 22 “unethical or questionably ethical studies” conducted by American and European researchers. These included, for example, a clinical experiment in the 1960s at the Willowbrook State School in which healthy mentally retarded children were infected with hepatitis, and the injection of cancer cells into patients at the Jewish Chronic Disease Hospital.
H. K. Beecher, “Ethics and Clinical Research,” New England Journal of Medicine 274, no. 24 (1966): 1354-60.
Research Ethics
Judicial Council of the American Medical Association Issues “Ethical Guidelines for Clinical Investigation”
At the 1966 Annual Convention of its House of Delegates, the American Medical Association endorsed the ethical principles set forth in the 1964 Declaration of Helsinki of the World Medical Association concerning human experimentation. These principles are consistent with those already included in the Principles of Medical Ethics of the AMA.
Technology
Left Ventricular Bypass Surgery Performed by Michael DeBakey
After a four-hour surgery, Dr. Michael DeBakey discovered that he could not restart his patient’s heart. He implanted an external heart pump which kept her alive for 10 days while her heart recovered. This surgery at the Baylor College of Medicine was the first successful use of the pump.
Research Ethics
Animal Welfare Act

The Animal Welfare Act is signed into law. It is the only Federal law in the United States that regulates the treatment of animals in research, exhibition, transport, and by dealers. Other laws, policies, and guidelines may include additional species coverage or specifications for animal care and use, but all refer to the Animal Welfare Act as the minimum acceptable standard. The Act is enforced by the USDA, APHIS, and Animal Care.
Professionalism and Ethics
American Medical Association Addresses Discrimination
The AMA House of Delegates adopts a resolution to give the Judicial Council the authority to expel constituent societies for race discrimination in membership policies. There is a reaffirmation of cooperation between the AMA and the National Medical Association (NMA) and the first mention by the AMA of seeking ways to increase the presence of African Americans in medicine. A provision allowing the AMA to expel any state society found guilty of discrimination passed in 1968.
1967
Technology
First Human-to-Human Heart Transplants Performed by Barnard and Kantrowitz
On December 3, 1967, Dr. Christiaan Barnard performed the first successful human heart transplant at the Groote Schubert Hospital in South Africa, placing him and the hospital in an international spotlight. The patient survived for 18 days; although the heart pumped strongly, he succumbed to infection secondary to immune suppression to prevent rejection.
Dr. Adrian Kantrowitz performed the world’s second transplant, and the first in the United States, at Maimonides Hospital in Brooklyn three days after Dr. Barnard’s surgery. The patient, an infant, survived for 6.5 hours. Kantrowitz would go on to be best known for the Intra-Aortic Balloon Pump.
End-of-Life Issues
Founding of St. Christopher’s Hospice in London

Cicely Saunders founds St. Christopher’s Hospice in London. As a nurse by profession, she focused on effective pain management and insisting that dying people needed dignity, compassion, and respect, as well as rigorous scientific methodology in the testing of treatments. She also believed that it was acceptable and even desirable to be honest with patients about their prognosis.
1968
End-of-Life Issues
Clinician-Patient Relationship
Definition of Death
An ad hoc committee at Harvard Medical School reexamined the definition of brain death and defined irreversible coma, or brain death. These definitions and guidelines would facilitate retrieval of organs for transplantation.
“A Definition of Irreversible Coma: Report of the Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain Death,” Journal of the American Medical Association 205 no. 6 (1968): 337-40.
Technology
Organ Donation
Uniform Anatomical Gift Act (UAGA) is formulated and recommended by the National Conference of Commissioners on Uniform State Laws for adoption by the states. All states in the United States rapidly passed relatively uniform laws based on the UAGA.
Ethical Guidelines for Organ Transplantation, Judicial Council of the American Medical Association
Reproductive Ethics
Religion and Ethics
Pope Paul VI issues Humanae Vitae
The last of Pope Paul VI’s encyclicals affirmed traditional Church moral teachings on the sanctity of life, the procreative role of conjugal relations and the rejection of artificial contraception. This, the last of Pope Paul’s encyclicals, was politically controversial as it did not accept the conclusions of his predecessor’s Pontifical Commission on Birth Control.
Professionalism and Ethics
American Medical Association Addresses Discrimination
The AMA House of Delegates adopts a resolution to give the Judicial Council the authority to expel constituent societies for race discrimination in membership policies. There is a reaffirmation of cooperation between the AMA and the National Medical Association (NMA) and the first mention by the AMA of seeking ways to increase the presence of African Americans in Medicine.
1969
Bioethics Organizations
Founding of The Hastings Center

The Hastings Center was founded by Daniel Callahan and Willard Gaylin, and was recognized as a nonprofit organization on August 28, 1969. Offices were originally located in Hastings-on-Hudson, N.Y. It was the first formal institution in the world established to pursue the study of ethical problems in medicine and biology. Within two years, The Hastings Center started publishing the Hastings Center Report, which explores ethical, legal, and social issues in medicine, health care, public health, and the life sciences. Six issues per year offer articles, essays, case studies of bioethical problems, columns on law and policy, caregivers’ stories, peer-reviewed scholarly articles, and book reviews.
Bioethics Organizations
Founding of the Society for Health and Human Values
The Society for Health and Human Values (SHHV) was established as a professional membership organization for persons committed to human values in medicine. The primary objective was to encourage and promote informed concern for human values as an essential, explicit dimension of education for health professionals.
End-of-Life Issues
Clinician-Patient Relationship
Publication of Elizabeth Kubler-Ross’ On Death and Dying
Inspired by her work with terminally ill patients and motivated by the lack of instruction in medical schools on the subject of death and dying, Kübler-Ross examined death and those faced with it. She described the stages of grief as: denial, anger, bargaining, depression, and acceptance.
Kubler-Ross, E., On Death and Dying (New York: Simon & Schuster, 1969).
1970
Reproductive Ethics
Hawaii and New York Legalize Abortion
New York City establishes an abortion program; approximately two thirds of the pregnancy terminations will be performed on patients not from New York State. The Department of Health brings in a statistician to collect data on the program. This data illustrates the safety of medically supervised abortions and was instrumental in the Supreme Court decision in Roe v. Wade.
J. Patter et al., “Two Years Experience in New York City with the Liberalized Abortion Law–Progress and Problems,” American Journal of Public Health 63, no. 6 (1973).
Reproductive Ethics
Publication of Daniel Callahan’s Abortion: Law, Choice and Morality
This was a groundbreaking and timely treatise on the legal, moral, and social issues surrounding the controversial topic of abortion. Such a discussion was extremely important as N.Y. and Hawaii legalized abortion and the Supreme Court would soon legalize abortion in all 50 states in the landmark Roe v. Wade decision. It was acclaimed at the time in book reviews as substantive and important and would establish Dan Callahan as an important voice in the societal debate about abortion.
D. Callahan, Abortion: Law, Choice and Morality (New York: Macmillian, 1970)

1971
Publication of John Rawls’ A Theory of Justice
John Rawls proposes a theory of justice that provides an alternative to utilitarian theories of justice and addresses concerns about the distribution of benefit. His theory is a form of social contract theory. While his work does not address health policy, it profoundly influences subsequent ethical analysis regarding the fair distribution of health care and other health-related interventions.
J. Rawls, A Theory of Justice (Harvard University Press, 1971)
Bioethics Organizations
Founding of The Kennedy Institute of Ethics
The Joseph and Rose Kennedy Institute of Ethics at Georgetown University was founded under the leadership of scientist, physician, and ethicist Andre Hellegers with support from the Kennedy Foundation. As an academic ethics center, it contributes to bioethics and other areas of practical ethics.
Arts and Ethics
Reproductive Ethics
End-of-Life Issues
Film “Who Should Survive” Created
A film about a newborn with Down Syndrome who is allowed to die is produced by the Kennedy Foundation. This film exposes a common practice in newborn nurseries and contributes to public dialogue on neonatal decision-making.
https://mn.gov/mnddc/ada-legacy/who-should-survive.html
Professionalism and Ethics
Publication of Rosemary Stevens’s American Medicine and the Public Interest
American Medicine and the Public Interest is a groundbreaking book on the history of specialization in medicine and its role in medical education and public policy.
R. Stevens, American Medicine and the Public Interest (New Haven: Yale University Press, 1971).
https://www.ucpress.edu/book/9780520210097/american-medicine-and-the-public-interest
1972
Research Ethics
Public Health Ethics
Tuskegee Syphilis Study Revealed

The study of “Untreated Syphilis in the Negro Male” was begun by the U.S. Public Health Service in 1932. In the mid-1960s, Peter Buxton, a PHS venereal disease investigator in San Francisco, found out about the Tuskegee study and expressed concerns to his superiors that it was unethical. The PHS formed a review committee but ultimately opted to continue the study with the goal of tracking participants until all had died so that autopsies could be performed and project data could be analyzed. Buxton then leaked the story to a reporter friend who passed it on to a fellow reporter. Jean Heller of the Associated Press broke the story in July 1972, prompting public outrage and forcing the study to shut down.
J. Heller, “Syphilis Victims in U.S. Study Went Untreated for 40 Years,” New York Times, July 26, 1972.
Informed Consent
Canterbury v. Spence
Canterbury v. Spence (464 F.2d. 772, 782 D.C. Cir. 1972) was a landmark federal case decided by the United States Court of Appeals for the District of Columbia Circuit that significantly reshaped malpractice law in the United States. It established the idea of “informed consent” to medical procedures.
https://biotech.law.lsu.edu/cases/consent/canterbury_v_spence.htm
Technology
Resource Allocation
End-Stage Renal Disease Act
In 1972, the United States Congress passed legislation authorizing the End Stage Renal Disease Program (ESRD) under Medicare. Section 299I of Public Law 92-603, passed on October 30, 1972, extended Medicare coverage to Americans if they had stage five chronic kidney disease and were otherwise qualified under Medicare’s work history requirements. The program’s launch was July 1, 1973. Previously, only those over 65 could qualify for Medicare benefits. This entitlement is nearly universal, covering over 90% of all U.S. citizens with severe chronic kidney disease.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4194691/
Feminist Ethics
Publication of Our Bodies, Ourselves

The Boston Women’s Health Collective publishes Our Bodies, Ourselves: A Book By and For Women. In 1969, twelve women at a women’s liberation conference in Boston who share information, personal stories, and their experiences with doctors, decided to put their knowledge into an accessible format. This information could be shared and would serve as a model for women who want to learn about themselves, communicate their findings with doctors, and challenge the medical establishment to change and improve the care women received. First published in a 193-page stapled format in 1970 called “Women and Their Bodies,” it was published more professionally in 1971, as “Our Bodies, Ourselves,” to emphasize women taking full ownership of their bodies. It was marketed commercially by Simon & Schuster in 1972. The book was revolutionary for its frank talk about sexuality and abortion, and for challenging the male obstetrician’s presumption of authority over women’s bodies.
Research Ethics
Publication of Jay Katz, Alexander Capron, and Eleanor Glass’s Experimentation with Human Beings: The Authority of the Investigator, Subject, Professions, and State in the Human Experimentation Process
This extraordinary compendium of information about cases of questionable research ethics offered authoritative source of information on the subject in the formative years of the field of bioethics.
J. Katz, A. Capron, and E. Glass, Experimentation with Human Beings: The Authority of the Investigator, Subject, Professions, and State in the Human Experimentation Process(New York: Russell Sage Foundation, 1972.)
https://www.russellsage.org/publications/experimentation-human-beings
1973
Reproductive Ethics
Abortion Is Legalized by the United States Supreme Court (Roe v. Wade, 410 U.S. 113)
This landmark decision of the U.S. Supreme Court ruled that the U.S. Constitution protects a pregnant woman’s liberty to choose to have an abortion without excessive government restriction. Doe v. Bolton, 410 U.S. 179, was a decision of the U. S. Supreme Court overturning the abortion law of Georgia. The Supreme Court’s decision was released on January 22, 1973, the same day as the decision in the better-known case of Roe v. Wade.
https://www.oyez.org/cases/1971/70-18
Ethics of Health Policy
Patient’s Bill of Rights
https://www.americanpatient.org/aha-patients-bill-of-rights/
Research Ethics
Public Health Ethics
Final Report of the Tuskegee Syphilis Study Ad Hoc Advisory Panel Is Submitted to the U.S. Public Health Service
The Advisory Panel described the study as ethically unjustified and recommended that a permanent national board be established with authority to regulate federally supported research involving human subjects.
https://biotech.law.lsu.edu/cphl/history/reports/tuskegee/complete%20report.pdf
Technology
Artificial Heart
The Artificial Heart Assessment Panel of the National Heart and Lung Institute of the National Institutes of Health issues a report of the Totally Implantable Artificial Heart. This panel was charged with assessing the social, ethical, and medical implications of a plutonium-powered, totally implantable artificial heart. Radiation risks ultimately advised against this technology despite enormous amounts of money spent to develop it.
H. P. Green, “An NIH Panel’s Early Warning,” Hastings Center Report 14, no. 5 (1984), 13-15.
https://www.jstor.org/stable/pdf/3561089.pdf
End-of-Life Issues
Clinician-Patient Relationship
Publication of Raymond Duff and A. G. Campbell’s Moral and Ethical Dilemmas in the Special-Care Nursery
In this New England Journal of Medicine article, Duff and Campbell describe the open process used in the neonatal ICU at Yale-New Haven Hospital to reach consensus about the treatment of catastrophically ill newborns. In many hospitals, these decisions were made behind closed doors with varying levels of involvement of parents. This article facilitated public debate and transparent decision-making.
R. S. Duff and A. G. Campbell, “Moral and Ethical Dilemmas in the Special-Care Nursery,” New England Journal of Medicine 289, no. 17 (1973): 890-4.
https://www.nejm.org/doi/full/10.1056/NEJM197310252891705
Professionalism and Ethics
First Edition of the Bibliography of Society, Ethics, and the Life Sciences Published by The Hastings Center
This is one of the first efforts to transform the fledgling field of bioethics into a legitimate professional field that shares a common literature. Authored by Sharon Sollitto and Robert M. Veatch.
https://www.amazon.com/Bibliography-society-ethics-life-sciences/dp/B0006VUXYQ
Informed Consent
End-of-Life Issues
Clinician-Patient Relationship
The Dax Cowart Case

Dax Cowart was severely burned by a propane gas explosion and fire in East Texas. He attempted to refuse treatments for his burns. Physicians in Dallas and Galveston disregarded his pleas on the grounds that he was incompetent. He argued passionately that he had the right to refuse the excruciatingly painful treatments for his burns, and that he had the right to die, though his case was not litigated. His story was the subject of a 1974 documentary, “Please Let Me Die.” He made a calm and rational argument for personal autonomy and his right to make his own treatment decisions.
https://jamanetwork.com/journals/jama/article-abstract/379390
End-of-Life Issues
Clinician-Patient Relationship
The Postma Case
The legal debate about euthanasia in the Netherlands is fueled by the Postma case in which a physician facilitated the death of her mother following repeated explicit requests for euthanasia is convicted. Dr. Postma was given a lenient sentence and the decision provided an opportunity for regulating euthanasia by acknowledging that a physician does not always have to keep a patient alive against his or her will when faced with pointless suffering.
J.A. Rietjens et al. “Two Decades of Research on Euthanasia from the Netherlands. What Have We Learnt and What Questions Remain?”Journal of Bioethic Inquiry 6, no. 3 (2009): doi:10.1007/s11673-009-9172-3. PMC 2733179.
https://link.springer.com/article/10.1007/s11673-009-9172-3
1974
End-of-Life Issues
Clinician-Patient Relationship
First Hospice in the United States Founded
Florence Wald, former Dean of the Yale School of Nursing, founds the first hospice in the United States—the Connecticut Hospice in Branford, Connecticut—marking the beginning of the palliative care movement in the United States.
Research Ethics
Ethics of Health Policy
Bioethics Commissions and Other Government Entities
National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research
The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, the first public national body to shape bioethics policy in the United States, was created as Title II of the National Research Act, which was signed into law by President Richard Nixon. The Commission, which was formed in the aftermath of the Tuskegee Syphilis Study, was charged with identifying ethical principles to be followed when conducting biomedical and behavioral research on humans and with establishing guidelines for the conduct of such research. https://bioethicsarchive.georgetown.edu/pcsbi/history.html
Privacy and Confidentiality
Tarasoff v. Regents of the University of California
In this case [17 Cal. 3d 425, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976)] the Supreme Court of California held that mental health professionals have a duty to protect individuals who are being threatened with bodily harm by a patient.
https://law.justia.com/cases/california/supreme-court/3d/17/425.html
Technology
Publication of Renée Fox and Judith Swazey’s The Courage to Fail. A Social View of Organ Transplants and Dialysis
Renee Fox and Judith Swazey combine their respective sociological and historical perspectives to explore how biomedical research has influenced medical practice in the United States through a focus on the development of organ transplantation. R. Fox and J. Swazey, The Courage to Fail. A Social View of Organ Transplants and Dialysis (reprinted: Cambridge University Press, 2012).
Genomics and Ethics
Recombinant DNA Advisory Committee (RAC)
The Recombinant DNA Advisory Committee (RAC) was established by the National Institutes of Health in 1974 to provide recommendations to the NIH Director and a public forum for discussion of the scientific, safety, and ethical issues related to basic and clinical research involving recombinant or synthetic nucleic acid molecules. The RAC and later its Human Gene Therapy Subcommittee helped to set the criteria for the evaluation of recombinant DNA protocols, including human gene therapies, which were examined by the RAC. In 2019, the NIH refocused the RAC into a role closer to its original mandate, which was to follow and provide advice on safety and ethical issues associated with emerging biotechnologies. These emerging areas of research include, but are not restricted to, technologies surrounding advances in recombinant or synthetic nucleic acid research. To reflect its broader outlook, the committee was renamed the Novel and Exceptional Technology and Research Advisory Committee (NExTRAC). The RAC documents, meeting materials, and webcasts have been archived and remain available on this website: https://osp.od.nih.gov/biotechnology/recombinant-dna-advisory-committee/
1975
Research Ethics
Asilomar Conference on Recombinant DNA

This influential conference was organized to discuss the potential biohazards and regulation of biotechnology. The participants included biologists, lawyers, and physicians. They developed voluntary guidelines aimed to ensure safety as recombinant DNA technology developed. The conference also brought cutting edge scientific research more into the public domain. The organizer, Paul Berg, would go on to win the 1980 Nobel Prize in Chemistry along with fellow organizers Walter Gilbert and Frederick Sanger.
P. Berg et al., “Summary Statement of the Asilomar Conference on Recombinant DNA Molecules,” PNAS 72, no. 6 (1975), 1981-84.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC432675/
Clinician-Patient Relationship
Publication of Jay Katz and Alexander Capron’s Catastrophic Diseases: Who Decides What?
This groundbreaking book examines human choice in the treatment of and research around catastrophic illness. It looks at patients, physicians, and investigators examining the pressures, conflicts, and decisions they confront, and examines emerging technologies from the perspective of facts and values.

J. Katz and A. Capron, Catastrophic Diseases: Who Decides What? (New York: Russell Sage Foundation, 1975)
https://www.amazon.com/Catastrophic-Diseases-Who-Decides-What/dp/0871544393
End-of-Life Issues
Clinician-Patient Relationship
Karen Quinlan

On April 15, 1975, at the age of 21, Karen Quinlan became unconsciousness after consuming alcohol and Valium at a party. She lapsed into a coma followed by a persistent vegetative state. Her parents asked that she be removed from her ventilator, which they believed constituted extraordinary means of prolonging her life. They filed suit in NJ Superior Court on September 12, 1975. See 1976 entry for In Re Quinlan decision.
The Karen Quinlan case: problems and proposals.
Professionalism and Ethics
Publication of LeRoy Walters’ Bibliography of Bioethics
This publication reinforces the development of bioethics as a scholarly field by assessing the existence of a new field, “bioethics,” and defining its literature.
L. Walters, Bibliography of Bioethics (Washington DC: Kennedy Institute Center for Bioethics, 1975).
Journals in Bioethics
Publication Begins of the Journal of Medical Ethics
This official journal of the Institute of Medical Ethics in the United Kingdom is an international journal encompassing the entire field of medical ethics, promoting ethical reflection and conduct in scientific research and medical practice.
Animal Rights
Publication of Peter Singer’s Animal Liberation: A New Ethics for Our Treatment of Animals (New York: Random House)
This influential book is viewed by the animal liberation movement to be the founding philosophical statement of its ideas. Singer popularized the term “speciesism” and argued that the interests of animals should be considered because of their ability to experience suffering.
P. Singer, Animal Liberation: A New Ethics for Our Treatment of Animals(New York: Random House, 1975.)
https://www.amazon.com/Animal-Liberation-Definitive-Classic-Movement/dp/0061711306
1976
Public Health Ethics
Ebola Outbreak
In 1976, the first cases of Ebola virus were discovered near the Ebola River in Zaire (now known as the Democratic Republic of Congo). From the moment of its discovery until the present day, the disease has been a focus of controversy as narratives about scientific triumphalism conflicted with anthropological observations of cultural imperialism in the guise of science. Although indigenous cultural beliefs and practices were blamed for preventing effective treatment of the disease, a major cause of the virus’s spread turned out to be improper sterilization of needles, i.e., so-called scientific medicine. The outbreak was ultimately contained by traditional epidemiological methods of contact tracing, isolation, and quarantine.
Science Mag: Part 1: Virologists tale Africa’s first encounter with Ebola
Science Mag: Part Two: Virologists tale Africa’s first encounter with Ebola
The Lacnelot: Ebola: Limits of correcting misinformation
Technology
Genomics and Ethics
Genentech Corporation Founded
Using recombinant E. coli, the company produced the first human protein, somatostatin, as a proof-of-concept in 1977, thereby laying the conceptual and industrial foundations of the modern biotechnology industry.
Gene.com: Genentech was founded more than, late venture capitalists
End-of-Life Issues
Clinician-Patient Relationship
Robert Veatch’s Publication of Death, Dying, and the Biological Revolution
This publication critiques the philosophical and ethical basis of the Ad Hoc Harvard Committee’s definition of brain death. Veatch also sets a precedent for ethics consultant by serving as an advisor to the Quinlans, thereby exemplifying one of the central claims of his book and of The Hastings Center: that cases like that of Karen Ann Quinlan raise fundamental ethical issues that should be analyzed by those trained in ethics and discussed by the public at large.
R. Veatch, Death, Dying, and the Biological Revolution (New Haven: Yale University Press, 1976).
Yalebooks: Death, Dying and Biological Revolution
End-of-Life Issues
Clinician-Patient Relationship
In Re Quinlan
The New Jersey Supreme Court decided that Joseph Quinlan, father of comatose ventilator dependent patient, Karen Ann Quinlan, may assert her right to privacy and that, pending review by a hospital ethics committee (HEC), that right is broad enough to encompass discontinuation of Karen Ann Quinlan’s ventilator support. The decision confirmed patients and surrogates legal right to refuse life sustaining medical interventions. It also publicized the concept of hospital ethics committees and catalyzed a national discussion of end-of-life issues, couched in terms of refusing “extraordinary,” or “heroic,” treatment.
In Re Quinlan, 70 N.J. 10; 355 A.2d 647 (1976)
End-of-Life Decision Issues
Clinician-Patient Relationship
“Orders Not to Resuscitate”
Two Harvard-affiliated teaching hospitals publish their policies on nonresuscitation decisions in a 1976 issue of the New England Journal of Medicine, initiating public discussion of the ethical issues surrounding these decisions.
https://www.nejm.org/doi/full/10.1056/NEJM197608122950705
Privacy and Confidentiality
Tarasoff v. Regents of the University of California
This 1976 California caseestablishes California psychiatrists’ duty to breach confidentiality, i.e., to warn specifically identified persons if their patients might harm these persons. It precipitated discussions of the duty to warn within the profession, in public forums, and in the nascent field of bioethics.
Tarasoff v. Regents of the University of California, 17 Cal. 3d 425, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
https://timeline.com/tanya-tarasoff-notify-law-7d43951cb004
International Ethics
United Nations Enacts International Covenant on Civil and Political Rights

Article 7 states: “No one shall be subjected to torture or to cruel, inhuman or degrading treatment or punishment. In particular, no one shall be subjected without his free consent to medical or scientific experimentation.” This lays the foundation for later international guidelines on experimentation on human subjects and provides a basis for condemning health care professionals’ participation in torture.
https://www.ohchr.org/en/professionalinterest/pages/ccpr.aspx
Journals in Bioethics
Journal of Medicine and Philosophy Founded by Edmund Pellegrino and H. Tristram Engelhardt Jr.
At its inception, the scope and focus of the field now known as bioethics was unclear. Some, like the editors of the trailblazing Journal of Medicine and Philosophy, conceived of it as a practical application of philosophy, and moral philosophy to medicine. Early editions of Tom Beauchamp and James Childress’s Principles of Biomedical Ethics shared this applied philosophy and moral philosophy conception. Later, their textbook treated the field as sui generis, a mix of practical guidelines, norms, and precepts with a history and focus influenced by and responsive to law, medicine, technology, religion, as well as moral philosophy, that came to be known as bioethics.
1977
International Ethics
Additional Protocol to the Geneva Conventions of 1977
This protocoldecrees it a violation of international humanitarian law to deliberately target or endanger wounded people, or civilian medical personnel, or equipment, or supplies in international conflicts, including colonial wars of national liberation. The protocol protects health care personnel, field hospitals, and other medical facilities rendering humanitarian assistance during international conflicts and wars of national liberation.
https://www.icrc.org/en/doc/resources/documents/misc/additional-protocols-1977.htm
End-of-Life Issues
Clinician-Patient Relationship
Superintendent of Belchertown v. Saikewicz
This 1977 Massachusetts case raises questions about a guardian’s substituted judgment, i.e., a decision by a one person on behalf of another person who is incapable of deciding for himself or herself, in a decision to refuse chemotherapy on behalf of a mentally disabled 67-year-old male patient. Drawing on the Quinlan case, decided the previous year, and then-recent articles in the medical literature (such as “Orders Not to Resuscitate”) the court found “no State interest sufficient to counterbalance a patient’s decision to decline life-prolonging medical treatment in the circumstances of this case, we conclude that the patient’s right to privacy and self-determination is entitled to enforcement. . . . and in view of the position of equality of an incompetent person in Joseph Saikewicz’s position, we conclude that the probate judge acted appropriately in this case.”
Superintendent of Belchertown State School v. Saikewicz, 373 Mass. 728, 370 N.E.2d 417, 1977 Mass. LEXIS 1129 (Mass. 1977).
https://law.justia.com/cases/massachusetts/supreme-court/1977/373-mass-728-2.html
1978
Professionalism and Ethics
Encyclopedia of Bioethics
In the 1970s, the name and scope of the field now known as “bioethics” was yet to be determined. The Hastings Center described itself as focusing on “ethics and the life sciences,” designating its scope as “pressing . . . ethical problems” in “the biological revolution, population explosion, and environmental crisis.” Other common characterizations were “health and human values,” “medicine and philosophy,” “medical ethics,” “medical humanities,” and “philosophy and medicine,” each describing the field and its scope slightly differently. In 1974, however, the Library of Congress had given the description “bioethics” canonical status, entering it as an official subject heading. Reflecting this, when the Kennedy Institute of Bioethics instituted a bibliographical service for the emerging field in 1975, it called it the “Bibliography of Bioethics,” and when the Institute published this four-volume, nearly 2,000-page book, it naturally titled it “The Encyclopedia of Bioethics,” delineating the anticipated content and disseminating what would become the name for the emerging field.
Reproductive Ethics
First In Vitro Fertilization (IVF) Baby
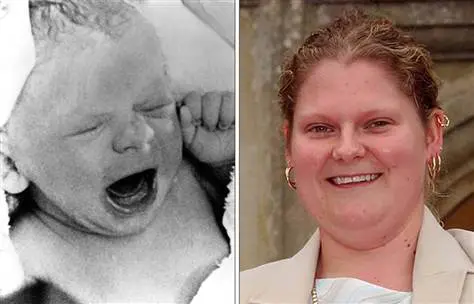
Louise Joy Brown was born in England on July 25, 1978. The new IVF techniques were developed by Patrick Steptoe, Robert Edwards (Nobel Laureate), and Jean Purdy. The media dubbed Louise a “test tube baby,” and IVF was immediately condemned by the Vatican.
NY Times: Test Tube Baby Born in US Joning Successes Around the World
Liberation or Oppression? Radical Feminism and In-Vitro Fertilization
USCCB: Begotten-not-made: A Catholic view of Reproductive Technology
International Ethics
Uniform Requirements for Manuscript Submissions
The Vancouver Group of medical journal editors, later the International Council of Medical Journal Editors (ICMJE) issues the “Uniform Requirements for Manuscript Submissions.” These require that authors confirm that their research has been conducted in accordance with the World Medical Association Declaration of Helsinki as a precondition for publication, thereby indirectly enforcing the Declaration.
http://www.icmje.org/recommendations/
Bioethics Commissions and Other Government Entities
President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research Established
The Commission’s ten reports published from 1981 to 1983 changed biomedical ethics, regulations, and laws. Among the most influential were: Defining Death (1981), Splicing Life (1982), Deciding to Forego Life-Sustaining Treatment (1983).https://bioethics.georgetown.edu/library-materials/digital-collections/us-bioethics-commissions/embed/#?secret=SJzrXua4kv
Research Ethics
Animal Rights
Three R Characterization of Alternatives to Animal Experimentation
As initially proposed by W. M. S. Russell and Rex Leonard Burch in their 1959 book, The Principles of Humane Experimental Technique, researchers were to strive to replace, reduce, and refine, experiments on animals. As modified in 1978 by the British Research Defense Society, the 3R principles for ethical experimentation on animals require researchers to use alternatives that would replace the use of animals, make reduction possible in the number of animals used, and reduce pain or distress experienced by the animals used in their experiments.
The 3 Rs: Replacement, Reduction and Refinement: Animal Welfare Throughout the World
1979
Research Ethics
Bioethics Commissions and Other Government Entities
The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research

This publication by the U.S. National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research introduced three basic principles for assessing the ethics of research on human subjects: respect for persons (researchers are to respect subjects as autonomous agents and thus seek informed voluntary consent from them), beneficence (researchers’ duty to mitigate harm to research subjects and promote their welfare) and justice (researchers’ duty not to exploit vulnerable populations by choosing subjects because they are convenient or compliant). These principles transformed the concept of consent in morality and law. In precedents dating to the Duke of York’s laws of 1665, consent had served to alleviate researchers’ responsibility for inflicting injury, death, or other harms, on anyone not consenting to participate in an experiment. The Belmont Principles changed the focus from harm prevention to subjects’ rights to be respected as autonomous agents.
Georgetown University: Belmont Report Anniversary & Oral History
Journals in Bioethics
Research Ethics
The Hastings Center Launches IRB: Ethics & Human Research

The first publication dedicated to discussion of human subjects research ethics. Renamed and expanded to become Ethics and Human Research in 2019.
The Hastings Center: Ethics & Human Research
Ethical Theory and Methods
Publication of Tom Beauchamp and James Childress’s The Principles of Biomedical Ethics
This textbook introduced a principles-based account of biomedical ethics, drawing on four principles similar to, but subtly different from, the Belmont principles: autonomy, beneficence, nonmaleficence, and justice. As of 2020, the book was in its 8th edition and remains an international bestseller and a formative influence in the field.
T. Beauchamp and J. Childress, The Principles of Biomedical Ethics(New York: Oxford University Press, 1979).
https://ethics.org.au/big-thinkers-thomas-beauchamp-james-childress/
1980
Journals in Bioethics
Journal of Bioethics
This international philosophical journal dedicated to the new field of bioethics is founded by two Australian utilitarian ethicists, Helga Kuhse and Peter Singer.
https://www.tandfonline.com/doi/abs/10.1179/hrge.13.1.74643l1853363678
Journals in Bioethics
Founding of Metamedicine
This international journal dedicated to the philosophy of medicine dealt with foundational issues about the nature of disease and health. As interest in these subjects waned it was renamed Theoretical Medicine in 1983. As of 1998 it has been published under the title Theoretical Medicine and Bioethics, tracking the eclipse of interest in the philosophy of medicine and the rise of interest in the broader, more encompassing field of bioethics.
https://www.springer.com/journal/11017
Research Ethics
Technology
Bayh-Dole Act Enacted
This act permits researchers to patent inventions developed with government funds and thereby incentivizing the growth of the biopharmaceutical industry.
https://autm.net/about-tech-transfer/advocacy/legislation/bayh-dole-act
Technology
Diamond v. Chakrabarty Decision
The U.S. Supreme Court rules that a genetically modified bacterium can be patented because it is the product of human ingenuity, which thereby sets a precedent for patents on other life forms, establishing a legal basis for intellectual property protection for the then-nascent biotechnology industry.
https://supreme.justia.com/cases/federal/us/447/303/
Professionalism and Ethics
Revisions in AMA’s Principles of Medical Ethics
From 1847 to 1979 the AMA’s codes of ethics presumed that clinical encounters involve benign scientifically trained physicians paternalistically caring for patients who had the responsibility of complying with their doctors’ orders. In its 1980 revision, Principle IV formally states that “A physician shall respect the rights of patients . . . and shall safeguard patient confidences within the constraints of the law.”
Current Opinions of the Judicial Council of the American Medical Association (Chicago: American Medical Association, 1981).
AMA Association: AMA Principals / Medical Ethics
Genomics and Ethics
Publication of Stephen Jay Gould’s The Mismeasure of Man

Stephen Jay Gould’s book disproved earlier scientific racist studies. Gould also openly criticized psychological testing, such as IQ tests, and the ranking of intelligence.
S. J. Gould, The Mismeasure of Man (New York: W. W. Norton, 1980).
Ethical Theory and Methods
Publication of Alistair MacIntyre’s After Virtue: A Study in Moral Theory
In this critique of the rationalistic individualism of Western moral theory from the enlightenment to the present day, MacIntyre argues for the resurrection of such Aristotelian virtues as honesty, loyalty, trustworthiness, and the social practices that instill and enforce them. The work is regarded as foundational to modern virtue theory.
A. MacIntyre, After Virtue: A Study in Moral Theory (Notre Dame, IN: University of Notre Dame Press, 1980).
https://undpress.nd.edu/9780268035044/after-virtue/
1981
Bioethics Commissions and Other Government Entities
End-of-Life Issues
Clinician-Patient Relationship
Defining Death: Medical, Legal, and Ethical Issues in the Determination of Death
The report from the President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research addressed the issue of definitions of death, including neurological criteria for death known as “brain death.” The Report called for a uniform definition of death based on a “total brain” standard. This standard defines death as the death of the entire brain of a person. The Commission sought to address a need for a legal definition of death which could incorporate advances made by new technologies that could perform necessary bodily functions. There was wide agreement between physician testifiers to the Commission that a definition of death which referred to irreversible loss of brain functions was required.
https://scholarworks.iupui.edu/handle/1805/707
Public Health Ethics
Sexual and Gender Ethics
Gay-Related Immunodeficiency (GRID), later redubbed AIDS
Acquired Immune Deficiency Syndrome is recognized by the U.S. Centers for Disease Control (CDC).
NY Times: New Homosexual Disorder Worries Health Officials
Reproductive Ethics
Religion and Ethics
Pope John Paul II’s Allocution
The Pope denounces the “autonomous power of self-affirmation” underlying much of contemporary bioethics as a corrupt ideal. He also reaffirms the Church’s traditional condemnation of abortion as sinful in his 1981 Papal exhortation, Familiars Consortio, “On the Role of the Christian Family in the Modern World.”
End-of-Life Issues
Advance Care Planning/Advance Directives
Clinician-Patient Relationship
Right to Refuse Life Sustaining Treatment Upheld by New York Courts
In the Matter of Philip K. Eichner, On Behalf of Joseph C. Fox, Respondent, v. Denis Dillon. Brother Joseph Fox, an 83-year-old member of a Roman Catholic religious order, suffered a cardiac arrest and was placed on a ventilator, sustaining him in a permanent vegetative state. A fellow of his religious order, Father Eichner, sought appointment as Brother Fox’s legal guardian to act on Brother Fox’s express statement that were he in a situation like Karen Ann Quinlan, he would have ventilator support discontinued. The Supreme Court of New York held that Brother Fox had a common-law right to decline treatment and that wishes, expressed prior to becoming incompetent, should be honored. In 1981, the New York Court of Appeals upheld this ruling, declaring that a patient’s right should not be lost when a patient becomes incompetent.
NY Times: Right-to-Die Decision News Analysis
Research Ethics
U.S. Food and Drug Administration Revises Human Subjects Regulations
The FDA revises its regulations relating to experiments involving human subjects.
FDA: Clinical Trials and Human Subjects
Professionalism and Ethics
Islamic Code of Medical Professional Ethics
Abdullah Al-Awadi, Abdul Rahman, and colleagues present papers to the First International Conference on Islamic Medicine Celebrating the Advent of the Fifteenth Century Hijri. Kuwait Ministry of Health: Kuwait, 1981. This Islamic Code is widely accepted in the Muslim world.
Islamic Code: Medical Ethics Kuwait Document
Professionalism and Ethics
Publication of Sidney Bloch and Paul Chodoff’s Psychiatric Ethics
This is a widely used book addressing ethical issues that arise in psychiatric practice.
S. Bloch and P. Chodoff, Psychiatric Ethics (New York: Oxford University Press, 1981). https://oxfordmedicine.com/view/10.1093/med/9780198839262.001.0001/med-9780198839262
Disability Ethics
International Ethics
United Nations General Assembly Proclaims 1981 the “Year of Disabled Persons”
The General Assembly calls for action regionally, nationally, and internationally to provide equal opportunities for people with disabilities, and to integrate people with disabilities more fully into society.
UN General Assembly: Rehabilitation and Prevention of Disabilities
1982
Research Ethics
Publication of William Broad and Nicholas Wade’s Betrayers of the Truth: Fraud and Deceit in the Halls of Science
This book by New York Times science journalists exposes unethical practices by scientists, thereby initiating an era of “fraud busting” aimed at research science.
W. Broad and N. Wade, Betrayers of the Truth: Fraud and Deceit in the Halls of Science (New York: Simon and Schuster, 1982).

Betrayers of Truth: Fraud and Deceit in the Halls of Science
Ethical Theory and Methods
Ethics Committees and Ethics Consultation
Publication of Albert Jonsen, Mark Siegler, and William Winslade’s Clinical Ethics: A Practical Approach to Ethical Decisions in Clinical Medicine
This book introduces the casuist “Four Box” method of structuring clinical ethics consultation. This pragmatic approach to clinical ethics consultation rapidly became the preeminent handbook for the field. It divided consults into four subjects: medical indications; patients’ preferences; patients’ quality of life; and contextual features like background information such as professional, familial, religious, financial, legal, and institutional factors relevant to the ethical and clinical decisions being considered.
A. Jonsen, M. Siegler, and W. Winslade, Clinical Ethics: A Practical Approach to Ethical Decisions in Clinical Medicine (New York: Macmillan Publishing, 1982).
Clinical Ethics: A Practical Approach to Ethical Decisions in Clinical Medicine, 8e
Technology
Research Ethics
Jarvik-7 Implanted

An experimental artificial heart,Jarvick-7, was implanted into an experimental subject, Barney Clark, at the University of Utah Hospital in December 1982. Mr. Clark survived for 112 days.
Artificial Heart 30 Years Later
End-of-Life Issues
Clinician-Patient Relationship
Publication of Eric Cassell’s article, Nature of Suffering and the Goals of Medicine
In this paper Cassell distinguishes pain, a physiological state, and suffering, which is a persons’ response to challenges that threaten their intactness as a complex social and psychological entity. He concludes that “Physicians’ failure to understand the nature of suffering can result in medical intervention that (though technically adequate) not only fails to relieve suffering but becomes a source of suffering itself.”
The Nature of Suffering and the Goals of Medicine
The Nature of Suffering and the Goals of Medicine
1983
Research Ethics
International Ethics
Comité National d’Ethique Commissioned
France becomes the first country in continental Europe to establish a National Consultative Ethics Committee for Life Sciences and Health. In conjunction with the new Council for International Organizations of Medical Sciences (CIOMS) guidelines, this served as a stimulus for local hospitals to establish committees to review research on human subjects. Laws regulating these committees were enacted in 1988.
Research Ethics
International Ethics
Proposed International Guidelines for Biomedical Research Involving Human Subjects
Issued by the Council of International Organizations of Medical Science (CIOMS) and the World Health Organization (WHO), the CIOMS guidelines modified the World Medical Association’s 1964 Declaration of Helsinki to address the outbreak of HIV/AIDs and to address the biopharmaceutical industry’s multinational field trials on human subjects, focusing on those conducted in the developing world.
End-of-Life Issues
Clinician-Patient Relationship
Publication of Deciding to Forego Life-Sustaining Treatment
This report by The President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research integrated the bioethical paradigm of shared physician-patient decision-making into the daily routine of American health care, extending this model to end-of-life decision-making. To facilitate adoption of their recommendations, they published model durable powers of attorney, and similar documents relating to issues in end-of-life decision-making that were soon widely adopted and used.
Technology
Research Ethics
Isolation of an AIDS Retrovirus
A French team at the Pasteur Institute isolates a retrovirus from a patient with AIDS in 1983 and sends a sample of this virus to Robert Gallo at the U.S. National Institutes of Health. Instead of acknowledging their collaborative efforts, each later claimed this discovery and the resulting brouhaha distracted from the importance of their joint achievement.
Technology
Genomics and Ethics
Splicing Life
Splicing Life, a report on the social and ethical issues of the genetic engineering in humans, was issued by the President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research in 1983, introducing the concept of “genetic engineering” into the biomedical and bioethical lexicon.
https://bioethics.georgetown.edu/documents/pcemr/splicinglife.pdf
Resource Allocation
Technology
The Hastings Center Working Group on Organ Transplantation
This group of scholars focused on reforming the Uniform Anatomic Gift Act to include a requirement that potential surrogates be asked to consider donating the organs of deceased; reviewed issues of equity in organ distribution, and accessed alternatives to current policy (e.g., market allocation; presumed consent). No formal report was issued, but members published over a dozen articles on these issues in such publications as the Journal of the American Medical Association, the New England Journal of Medicine, and other influential medical journals.
Hastings Briefing Book: Organ Transplantation
1984
International Ethics
Bioethics Commissions and Other Government Entities
China Establishes a Bioethics Advisory Committee
China’s Ministry of Health sets up an advisory committee on bioethics in 1984 following the first publication of a Chinese textbook on bioethics the previous year. This is the first official recognition of bioethics as a field and initiates Chinese participation in international bioethics and acceptance of some international research ethics regulations.
Research Ethics
Privacy and Confidentiality
Sexual and Gender Ethics
Publication of Ronald Bayer, Carole Levine, and Thomas Murray’s Guidelines for Confidentiality in Research on AIDS
In this article, published in The Hastings Center journal IRB: Ethics and Human Research, the authors recommend two dozen guidelines for institutional review boards to use when reviewing research proposals involving people with HIV/AIDS, a group vulnerable to social stigmatization and discrimination. The guidelines are “designed to provide the basis for the cooperation of the research community and the subjects of AIDS research” in order to “afford the fullest degree of protection for confidentiality compatible with sound scientific research.”
https://www.jstor.org/stable/3564421?seq=1#metadata_info_tab_contents
Research Ethics
Professionalism and Ethics
Organizational Ethics
HIV/AIDS Research
Papers published in Science demonstrate that the HIV-1 virus isolated by the Pasteur Institute is responsible for Acquired Immune Deficiency Syndrome (AIDS). Robert Gallo of the National Institutes of Health was lead author. Instead of celebrating a successful international collaboration, the Pasteur Institute and the NIH and various scientists wrangled for years over who deserves credit for the discovery that the HIV retrovirus causes AIDS.
NIH Researchers Recall the First Years of AIDS
Robert Gallo at the Center of the History of HIV
End-of-Life Issues
Medical Aid in Dying
Clinician-Patient Relationship
Decision against Prosecution for Mercy Killing in the Netherlands
Accepted by the Dutch Supreme Court in the 1984 Alkmaar Case, the Court recognized an agreement negotiated by Dutch prosecutors and the Royal Dutch Medical Society that allowed a physician to avoid prosecution for euthanasia—prescribing a fatal dose of medication to a dying patient—provided that the physicians informed prosecutors of the details involving their euthanasia of a patient.
NY Times: Dutch Court acts on Right-to-Die
Background about Euthanasia in The Netherlands
Environmental Ethics
Ethical Theory and Methods
Publication of Hans Jonas’ The Imperative of Responsibility: In Search of an Ethics for the Technological Age
Precautionary Principle (Vorsorgeprinzip) introduced into English language bioethics and the environmental ethics lexicon in this 1984 book. This principle would become central to the worldwide environmental ethics movement.
H. Jonas, The Imperative of Responsibility: In Search of an Ethics for the Technological Age (Chicago: University of Chicago Press, 1984).
The Imperative of Responsibility: In Search of an Ethics for the Technological Age
Resource Allocation
U.S. National Organ Donor Transplantation Act of 1984
This Act prohibits the sale or purchase of human organs and establishes the Organ Procurement and Transplantation Network (OPTN) to be operated by a private, nonprofit organization under federal contract. (The United Network for Organ Sharing (UNOS) later received this contract.). This act precluded market solutions to organ shortages and makes organ donation (“the gift of life,” so-called) the national model for organ procurement. It also commissioned a Task Force on Organ Transplantation, which issued its report in 1986.
National Organ Transplant Act (1984 Pub.L. 98–507), approved October 19, 1984, and amended in 1988 and 1990.
Organ Transplant’s Problem In America: 30 Years Since The National Organ Transplant Act Was Passed
Professionalism
Doctor-Patient Relationship
Informed Consent
Publication of Jay Katz’s The Silent World of Doctor and Patient

In this book Katz described the paternalism of the traditional physician-patient relationship and recommended a form of shared decision making.
J. Katz, The Silent World of Doctor and Patient (Baltimore: The Johns Hopkins University Press, 1984).
The Silent World of Doctor and Patient by Jay Katz, MD
1985
Bioethics Pedagogy
Bioethics Syllabus Exchange Repository Established
Established at The Kennedy Institute of Ethics in 1985 to encourage the development of courses in the evolving field of bioethics.
Bioethics Syllabus Exchange Repository
Research Ethics
Animal Rights
First International Guiding Principles for Biomedical Research Involving Animals
Issued by the Council of International Organizations of Medical Sciences (CIOMS) in 1985.
International Guiding Principles for Biomedical Research Involving Animals
Bioethics Organizations
Professionalism and Ethics
Society for Bioethics Consultation (SBC)
A steering committee proposes the first society focused exclusively on clinical ethics consultation. The SBC was chartered as a nonprofit in 1986. In 1998, it merged with the American Society for Bioethics and the Society for Health and Human Values to form the American Society for Bioethics and the Humanities (ASBH).
Texas Archival Resources Online
Sexual and Gender Ethics
Ethics of Health Policy
Public Health Service Task Force Report on Women’s Health Issues
The first federal effort to examine women’s health issues which recognizes that women have different health issues than men. It led to the inclusion of women in human subjects research.
Report of the Public Health Service Task Force on Women’s Health Issues
Ethics of Health Policy
Social Justice
Heckler Report
Report of the Secretary’s Task Force on Black and Minority Health resulted in the creation of the Office of Minority Health in the Office of Health and Human Services.
Report of the Secretary’s Task Force on Black and Minority Health
1986
International Ethics
Publication of Raanan Gillon’s Philosophical Medical Ethics
This text, based on a series of articles in the British Medical Journal, introduced Beauchamp and Childress’s four principles approach, with attention to the scope of their application for a European and Commonwealth audience.
R. Gillon, Philosophical Medical Ethics(New York: Wiley, 1986).
Resource Allocation
Report of the Task Force on Organ Transplantation: Organ Transplantation: Issues and Recommendation
This national task force was established by the 1984 National Organ Transplant Act. Its several recommendations included recognizing “each donated organ . . . [as] a national resource to be used for the public good,” involving the public in setting the criteria for organ allocation and implementing required request-routine inquiry policies which thereby precluded the market allocation of solid organs.
Task Force on Organ Transplantation: Organ Transplantation: Issues and Recommendation (U.S. Department of Health and Human Services, Public Health Service, Health Resources and Services Administration, Office of Organ Transplantation, April 1986).
Donation & Transplantation History
End-of-Life Issues
Clinician-Patient Relationship
Bouvia v. Superior Court, Calif. Appeals Court

Elizabeth Bouvia, a 28-year-old woman with cerebral palsy and arthritis, and who was quadriplegic, had expressed a desire to die, refused oral feeding, and sought to remove the feeding tube her physicians had inserted, an action supported by the hospital’s ethics committee based on what was deemed a suicidal ideation. After the trial court found that she was not terminally ill and refused her request, the California Appellate court held that even if her condition was not terminal, her right to refuse treatment was encompassed by the U.S. Constitution’s right of privacy as well as that of the California constitution and the previous California appellate cases, Barber (1983) and Bartling (1984). The case was the first to find that right of privacy encompasses refusal of life sustaining treatment in the form of artificial nutrition and hydration for a patient with a nonterminal condition, though Ms. Bouvia eventually accepted oral nutrition and hydration and lived for decades afterward.
Bouvia v. Superior Court, 179 Cal.App.3d 1127, 225 Cal.Rptr. 297 (Cal.App. 2 Dist.1986).
Court Record: ELIZABETH BOUVIA v. THE SUPERIOR COURT OF LOS ANGELES COUNTY
Ethical Theory and Methods
Publication of H. Tristram Engelhardt’s The Foundations of Bioethics
Engelhardt critiqued the regnant bioethics paradigm for its failure to establish moral norms and offered a foundational analysis. Engelhardt’s vision, along with many philosophers and others trained by him, would have a significant impact in U.S. bioethics. The second edition of this book (1996) presents a radical revision of his views on finding a foundation for bioethics.
H. Tristram Engelhardt, The Foundations of Bioethics (New York: Oxford University Press, 1986).
Informed Consent
Publication of Ruth Faden and Tom Beauchamp’s (with Nancy King) A History and Theory of Informed Consent
Faden, Beauchamp, and King examine the history and theory of the philosophical foundations and origin and development of the U.S. legal doctrine of consent and refusal of medical treatment and participation in research, covering landmark cases including Schloendorff, Salgo, and Canterbury.
R. Faden and T. Beauchamp (with N. King), A History and Theory of Informed Consent (New York: Oxford University Press, 1986).
A History and Theory of Informed Consent
International Bioethics
China’s First National Conference on Medical Ethics
The conference adopted as the fundamental principle of Chinese medical ethics, the precepts: “Rescue the dying, heal the wounded, prevent illness, cure disease, implement revolutionary humanism, and maintain the health of the people wholeheartedly.” The focus of the conference was ethical issues raised by advanced biomedical technologies, such as life-sustaining technology, assisted reproductive technology, and organ transplantation technology.
ABA Country Report for China 2003
International Bioethics
Western Bioethics Introduced to China
A first edition of philosopher Qiu Renzong’s book Bioethics (Shanghai People’s Press, 1986) quickly sold out 50,000 copies.
Ethics prize goes to Chinese scientist
1987
End-of-Life Issues
Clinician-Patient Relationship
Publication of The Hastings Center’s Guidelines on the Termination of Life-Sustaining Treatment and the Care of the Dying

This publication was the first comprehensive consensus-based ethics guidance on end-of-life care. It provided guidelines for health care professionals on the decisions faced by dying patients, their families, and other caregivers. In the introduction to the second edition, the authors reflected on the first edition: “This groundbreaking book, whose principal author was Susan M. Wolf, was widely consulted and influential in the U.S. and internationally. It was cited by Justice O’Connor in the U.S. Supreme Court’s Cruzan decision and helped shape the current ethical and legal framework for medical decision-making and end-of-life care.”
Berlinger, N., Jennings, B., and Wolf, S. M., 2nd ed., The Hastings Center Guidelines for Decisions on Life-Sustaining Treatment and Care Near the End of Life (Oxford University Press, 2013).
Research Ethics
Publication of Benjamin Freedman’s Equipoise and the Ethics of Clinical Research
Freedman first defined and elaborated the use of the term “equipoise” in research–the position of uncertainty that justifies ethically prospective clinical trials. The concept would become an essential element of research ethics.
Freedman, B., “Equipoise and the Ethics of Clinical Research,” New England Journal of Medicine 317 (1987): 141-45.
Equipoise and the ethics of clinical research
Resource Allocation
Publication of Daniel Callahan’s Setting Limits: Medical Goals in an Aging Society
This publication presents an influential and controversial discussion of the health needs of the aging and of health care rationing. Callahan brought to task the U.S. health care system for its emphasis on technological prolongation of life at the cost of quality of life, which would result in disproportionate societal resource allocation on extension of life. He called for focusing technological advancements and health care provision on the aim of quality of life for the elderly rather than quantity.
D. Callahan, Setting Limits: Medical Goals in an Aging Society(New York: Simon & Schuster, 1987).
Setting Limits: Medical Goals in an Aging Society
Reproductive Ethics
Ethics and Religion
Donum Vitae: Instruction on Respect for Human Life in Its Origin and on the Dignity of Procreation: Replies to Certain Questions of the Day
Catholic Church, Congregation for the Doctrine of the Faith, Donum Vitae: Instruction on Respect for Human Life in Its Origin and on the Dignity of Procreation: Replies to Certain Questions of the Day (also known as the Vatican Instruction on the New Reproductive Technologies), responded to moral questions raised by technical interventions on human procreation and the relationships between moral law and civil law in terms of the respect due to human embryos and fetuses and the legitimacy of techniques of artificial procreation.
Bioethics Organizations
International Ethics
Founding of the European Society for the Philosophy of Medicine and Healthcare (ESPMH)
Philosophers, physicians, ethicists and others founded the European Society for the Philosophy of Medicine and Healthcare in the Netherlands. It hosts an international conference and publishes the journal, Medicine, Health Care and Philosophy as a “forum for international exchange of research data, theories, reports and opinions on bioethics, and the philosophy of medicine and health care.”
European Society for Philosophy of Medicine and Healthcare
Arts and Ethics
Sexual and Gender Ethics
Publication of Randy Shilts’s And the Band Played On: Politics, People, and the AIDS Epidemic

Randy Shilts, a reporter for the San Francisco Examiner, compellingly and meticulously documented the history of the acquired immune deficiency syndrome (AIDS) epidemic caused by human immunodeficiency virus (HIV). The devastation of what was then a fatal disease was aided by an inadequate societal response due apathy, incompetence, political forces, and discrimination. The book was adapted into a film in 1993.
R. Shilts, And the Band Played On: Politics, People, and the AIDS Epidemic(New York: St. Martin’s Press, 1987).
And the Band Played On Politics, People, and the AIDS Epidemic, 20th-Anniversary Edition
1988
Reproductive Ethics
Baby M, New Jersey Supreme Court
William and Elizabeth Stern contracted with Mary Beth Whitehead to act as a gestational surrogate using artificial insemination with Mr. Stern’s sperm, to be paid $10,000, and in exchange to give up the child and parental rights to the Sterns. The New Jersey Supreme court found that surrogacy contracts were void as against public policy, restored her rights, and remanded the case to the trial court to determine the child’s best interests. The trial court awarded custody to Mr. Stern as father and visiting rights to Ms. Whitehead, as mother, though the child, when she reached adulthood, would designate Ms. Stern as her mother through adoption. This first state court deliberation about surrogacy contract would be followed by others that would recognize the validity of surrogacy contracts and enforce their provisions.
Baby M, Matter of 109 N.J. 396, 537 A. 2d 1227 (N.J.1988).
Supreme Court of New Jersey: Matter of Baby M.
Resource Allocation
Oregon Medicaid Priority Setting Project
Oregon Senate President and emergency medicine physician John Kitzhaber, MD, develops the Oregon Medicaid Priority Setting Project, which would eventually form the basis for the eventual Oregon Health Services Commission and the Oregon Health Plan. The Commission would use an elaborate set of methods to establish a list of conditions and services in order of funding priority, creating what plan designers called “the world’s first prioritized list of health services,” and prompting widespread concerns related to “rationing.”
The Oregon Health Plan: An Historical Overview
Oregon’s Experiment with Prioritizing Public Health Care Services
Health care in common: setting priorities in Oregon
Arts and Ethics
Publication of Arthur Kleinman’s The Illness Narratives: Suffering, Healing and the Human Condition
Arthur Kleinman, a psychiatrist and anthropologist, argues that rich narrative descriptions of patient history and experience can help to address the fundamental and existential issues of meaning of the illness medically and personally.
A. Kleinman’s The Illness Narratives: Suffering, Healing and the Human Condition (New York: Basic Books, 1988).

The Illness Narratives: Suffering, Healing and the Human Condition
Arts and Ethics
Publication of Howard Brody’s Stories of Sickness
Family physician and philosopher Howard Brody, using literature and non-fiction essays shows how shared and understood narratives help physicians to heal – and patients to be healed – as an essential element of the therapeutic relationship. He advocates both for the power of stories as a diagnostic and therapeutic adjunct, as well as an approach to contextualize the ethical issues that arise in medical care.
H. Brody’s Stories of Sickness (New Haven: Yale University Press, 1988).
Journals in Bioethics
International Bioethics
Ethik in der Medizin
German language journal in bioethics begins publication.
Official Journal of the German Academy of Ethics in Medicine
Ethical Theory and Methods
Publication of Albert Jonsen and Stephen Toulmin’s The Abuse of Casuistry: A History of Moral Reasoning
Jonsen and Toulmin recount the history of casuistry, a form of moral reasoning and discernment based on analysis of cases and comparisons to other paradigmatic cases. Casuistry’s ascendance reached its zenith in the 16th and early 17th centuries (and is still used in law schools as a pedagogical format). The authors acknowledge and describe its past abuses but propose its revival and proper use as a powerful technique to aid in resolution of contemporary medical ethical issues.
A. Jonsen and S. Toulmin, The Abuse of Casuistry: A History of Moral Reasoning (Berkeley: University of California Press, 1988).
https://www.ucpress.edu/book/9780520069602/the-abuse-of-casuistry
1989
Research Ethics
Research Ethics Federal Regulatory Developments
The year 1989 was marked by several research ethics federal regulatory developments. U.S. public health agencies amended their definitions of misconduct and established new requirements of scientific integrity to be assured through scientific integrity review. The National Institutes of Health established requirement for responsible conduct of research education for graduate students on training grants. These newly promulgated definitions and requirements heightened the responsibilities of those researchers funded through NIH to adherence to the established research ethics regimen and placed a responsibility on research universities to educate the scientists in training similarly funded.
Ethical Theory and Methods
Publication of Allen Buchanan and Dan Brock’s Deciding for Others: The Ethics of Surrogate Decision Making
This analysis of the philosophical theory and practical implications of making decisions for those who become incapacitated or never had the capacity to make decisions became the foundational text of surrogate decision making.
A. Buchanan and D. Brock, Deciding for Others: The Ethics of Surrogate Decision Making (New York: Cambridge University Press, 1989).
Deciding for Others: The Ethics of Surrogate Decision Making
Feminist Ethics
Publication of Rosemary Tong’s Feminist Thought: A More Comprehensive Introduction
In Feminist Thought, Tong provides an overview of the major traditions of feminist theory and characterizes the role that gender can play in navigating ethical conflicts. She identifies the need for decisions, policies and innovations to be critically reviewed in consideration of the effects on women and the lives they live. Feminist Thought addresses many issues, including reproductive technologies, abortion, gender discrimination, and political and economic disparities.
R. Tong’s Feminist Thought: A More Comprehensive Introduction(Boulder, CO: Westview Press, 1989).
Feminist Thought: A More Comprehensive Introduction
1990
End-of-Life Issues
Clinician-Patient Relationship
Cruzan, U.S. Supreme Court

In 1983, Nancy Beth Cruzan required cardiopulmonary resuscitation after an automobile accident. She never regained consciousness. While she was weaned off a ventilator, her parents asked to have her feeding tube withdrawn. The hospital refused the request based on Missouri’s requirement for clear and convincing evidence of a patient’s wishes before withdrawing artificial nutrition and hydration. After Missouri prevailed in the state Supreme Court, the Cruzan’s appeal to the U.S. Supreme Court, which holds that the Constitution’s 14th Amendment due process liberty interest supports an individual’s right to refuse life sustaining medical treatment, including artificial nutrition and hydration; the court finds that Missouri may require the clear and convincing evidentiary standard of the patient’s wishes; evidence of her wishes is subsequently uncovered and Nancy Cruzan is allowed to die. The Cruzan case, the first U.S. Supreme Court decision on ethical issues at end of life, established a federal 14th Amendment due process liberty interest to be free of unwanted medical treatment, to which all federal and state courts must adhere.
Cruzan by Cruzan v. Director, Missouri Dept. of Health, 497 U.S. 261, 110 S.CT. 2841, 111 L.ED.2D 224 (1990)
Cruzan by Cruzan v. Director, Missouri Dept. of Health
Advance Care Planning/Advance Directives
End-of-Life Issues
Clinician-Patient Relationship
Patient Self-Determination Act of 1990
Spurred by the Cruzan case, Congress passes the Patient Self-Determination Act, requiring health care institutions to provide information about advance health care directives to adult patients upon their admission to the healthcare facility. Advance directives initially had little effect on end-of-life care, but later studies would show that patients with advance directives would be more likely to have their wishes respected. The Act encourages adults who tend not to complete advance directives to do so.
Patient Self-Determination Act, Omnibus Budget Reconciliation Act of 1990, P.L. 101-508, Nov. 5, 1990. Effective, Dec. 1, 1991.
Patient Self-Determination Act, Omnibus Budget Reconciliation Act of 1990
Research Ethics
Moore, California Supreme Court
A physician-scientist had a patient under treatment return repeatedly so they could harvest the patient’s blood cells for their research without disclosing the purpose of the visits or research. The physician-scientist then patented the patient’s cell line. The California Supreme Court held that the physician-scientist has a fiduciary duty and an informed consent requirement to disclose personal research or economic interests that may affect judgment, but the patient did not have a property interest in the patented cells. The case would have implications for regulatory and ethical considerations concerning research-related informed consent and intellectual property that would be revisited again in the discussion of cells derived in 1951 from cancer patient Henrietta Lacks.
Moore v. Regents of University of California, 271 Cal.Rptr 146, 793 P.2d 279 (Cal.1990)
Moore v. Regents of University of California
End-of-Life Issues
Clinician-Patient Relationship
Publication of Lawrence Schneiderman, Nancy Jecker, and Albert Jonsen’sArticle on Medical Futility
The authors proffered a quantitative definition of medical futility (an intervention having been “useless the last 100 times” it was attempted) as a practical clinical consensus of when the intervention is not ethically required.) Lauded and assailed both ethically and statistically, the article remains a landmark in the ethical consideration of when, if ever, an arguably potentially life prolonging medical intervention can be refused or not offered.
Schneiderman, L. J., Jecker, N. S., and Jonsen, A. R., “Medical Futility: Its Meaning and Ethical Implications,” Annals of Internal Medicine 112, no. 12 (1990): 949-54.
Medical futility: its meaning and ethical implications
End-of-Life Issues
Clinician-Patient Relationship
Professionalism and Ethics
Decisions near the End-of-Life Initiative
Medical education and practice change program led by Mildred Solomon, EdD and Bruce Jennings, MA that surveyed practicing clinicians, and informed by those findings, took bioethics into the field—reaching 40,000 providers in 237 health care institutions in 20 states from 1990 to 1996 and, through media coverage, the larger public as well. It was offered by the Education Development Center (EDC) of Newton, MA, in cooperation with The Hastings Center, the American Medical Association, the American Hospital Association, and the American Bar Association. Its work would reach end-of-life care across the United States, and would engender and inform subsequent programs such as the American Medical Association’s Education for Physicians on End-of-Life Care (EPEC) project (1998-present), a train-the-trainer program led by Linda Emanuel, MD, PhD, and subsequently by Joshua Hauser MD, now renamed the Education on Palliative and End-of-Life Care (EPEC) Program offered through Northwestern University that would reach thousands of trainers, and in turn, hundreds of thousands of health care providers.
Solomon, M. Z., et al., “Toward an Expanded Vision of Clinical Ethics Education: From the Individual to the Institution,” Kennedy Institute of Ethics Journal 1, no. 3 (1991): 225-45.
Toward an Expanded Vision of Clinical Ethics Education: From the Individual to the Institution
Medical Aid in Dying
End-of-Life Issues
Clinician-Patient Relationship
Jack Kevorkian Assists in Medical Death (by Suicide) of Janet Adkins

Jack Kevorkian, MD, a Michigan pathologist and advocate for euthanasia and medically assisted death, used a “machine” he had created that allowed Janet Adkins, a 54-year-old Alzheimer’s patient from Oregon, to self-administer thiopental, automatically followed by potassium chloride, causing cardiopulmonary arrest. Dr. Kevorkian would be acquitted of criminal charges from his actions in this case and in three other subsequent prosecutions. His attorney claimed he had been involved in 130 similar deaths. Dr. Kevorkian was successfully prosecuted for second degree homicide after he had directly administered medication to cause the death of Atrophic Lateral Sclerosis (ALS) patient Thomas Youk in 1998. Dr. Kevorkian died in 2011, four years after his release from prison.
Schneider, K., “Dr. Jack Kevorkian Dies at 83; A Doctor Who Helped End Lives,”
New York Times, June 3, 2011.
Dr. Jack Kevorkian Dies at 83; A Doctor Who Helped End Lives,
Genomics and Ethics
Human Genome Project
The U.S. National Institutes of Health and the Department of Energy initiated a $20 billion project in 1990 to sequence and map all human genes, known as the human genome. This project was led from the NIH by James Watson. The project’s aims were to determine the sequence of all the bases in human genome DNA; to map locations of genes for major sections of all human chromosomes; and to produce linkage maps through which inherited traits and genetic diseases can be identified. A nearly complete sequence of the three billion DNA base pairs was published in 2001, and the full sequence was completed and published in April 2003 by the International Human Genome Sequencing Consortium of over 2,000 researchers, with far fewer genes (about 20,000) than surmised. A major component of the Human Genome Project was devoted to the analysis of the ethical, legal, and social implications (ELSI) of the genomic discoveries and recommendations for policy.
Genomics and Ethics
First Human Gene Therapy Clinical Trial
The first gene therapy trial included two girls with adenosine deaminase (ADA) deficiency, a genetic immunological disease, and was done at the National Institutes of Health Clinical Center by W. French Anderson, MD, Kenneth Culver, MD, and Michael Blaese, MD, through the National Heart, Lung, and Blood Institute and the National Cancer Institute. Virally delivered therapy would be reexamined after the death of University of Pennsylvania research subject Jesse Gelsinger in 1999, though research in genetically altering somatic cells as a promising treatment has accelerated since the discovery of CRISPR-Cas9 (clustered regularly interspaced short palindromic repeats) and other related interventions.
Making History with the 1990 Gene Therapy Trial
Disability Ethics
Americans with Disabilities Act of 1990

The Americans with Disabilities Act is a U.S. comprehensive civil rights law passed by Congress that addresses the rights of people with disabilities, prohibiting discrimination in employment, public services, and public accommodations, among other areas. The Equal Employment Opportunity Commission would have the authority to regulate and enforce its provisions. The passage of this legislation paralleled an emerging area of bioethics focus, disability studies, pioneered by Adrienne Asch, PhD and Eric Parens, PhD, among others.
End-of-Life Issues
Clinician-Patient Relationship
Publication of Daniel Callahan’s What Kind of Life? The Limits of Moral Progress
Callahan critiques society’s technological imperative in medicine of the maintenance of health and the extension of life at all costs with insufficient attention to the telos of human life and health: the kind of life we should live. He asks the ancient question again: What in the modern medical technological age constitutes the good life?
D. Callahan, What Kind of Life? The Limits of Moral Progress(New York: Simon & Schuster, 1990).
What Kind of Life? The Limits of Moral Progress
Resource Allocation
Publication of Paul Menzel’s Strong Medicine: The Ethical Rationing of Health Care
Menzel makes the case for an ethical basis on which to ration beneficial health care through analyses of the Hippocratic tradition and the concept of prior consent that a reasonable person would be presumed to give should benefits need to be allocated justly among those who might need them at some future time.
P. Menzel, Strong Medicine: The Ethical Rationing of Health Care(New York: Oxford University Press, 1990).
Strong Medicine: The Ethical Rationing of Health Care
Professionalism and Ethics
Publication of Gregory Pence’s Classic Cases in Medical Ethics: Accounts of Cases That Have Shaped Medical Ethics, with Philosophical, Legal and Historical Backgrounds
This textbook includes major cases, both legal and medical, that have been newsworthy and advanced the national discussion of bioethics. The Quinlan and Cruzan legal cases, and Dax Cowart, a famous case that was never litigated, are examples.
G. Pence, Classic Cases in Medical Ethics: Accounts of Cases That Have Shaped Medical Ethics, with Philosophical, Legal and Historical Backgrounds(Boston: McGraw Hill, 1990).
Arts and Ethics
Awakenings, Columbia Pictures

Academy award nominated film based on the story of a physician working at a Bronx hospital with patients in a catatonic state from an epidemic that caused encephalitis. He finds that they awaken with L-dopa, but this breakthrough is shattered with the gradual realization by the physician that these effects will be only temporary as the disease becomes less responsive even to increased drug doses, and that the patients also realize their period of regained consciousness will be limited. The film is based on a memoir by physician and writer Oliver Sacks published in 1973.
Awakenings. Columbia pictures. 1990.
1991
Research Ethics
Bioethics Commissions and Other Government Entities
Publication of the “Common Rule”
The Federal Policy for the Protection of Human Subjects (“Common Rule”) was published. It was codified in regulations. The HHS regulations, 45 C.F.R., part 46, have four subparts: (A) the Federal Policy or “Common Rule,” (B) additional protections for pregnant women, (C) additional protections for prisoners, and (D) additional protections for children.
Federal Policy for the Protection of Human Subjects (‘Common Rule’)
Reproductive Ethics
Planned Parenthood of SE Pa., U.S. Supreme Court
The U.S. Supreme Court upheld its essential holding of its Roe v. Wade decision of 1973, abandoning its right of privacy analysis, but grounding the rights of pregnant women before viability in the 14th Amendment due process protections. Post-viability state restrictions such as a waiting period were permitted as long as the state regulation did not constitute an “undue burden,” such as requiring spousal notification. The plurality decision indicated that though Roe was still controlling U.S. legal precedent for the country, further proposed state restrictions would continue to challenge the strength of the Roe precedent.
Planned Parenthood of SE Pa. v. Casey 505 U.S. 833 (1992)
Planned Parenthood of Southeastern Pennsylvania v. Casey
Professionalism and Ethics
Publication of Eric Cassell’s The Nature of Suffering and the Goals of Medicine
Cassell issued a call to action for the profession of medicine to address not just the physical symptoms of illness, but also patient suffering, defined as “the state of severe distress associated with events that threaten the intactness of the person” by learning about the patient as person, including their past history, and their values, wishes, and concerns. His vision of physician as healer of illness and salver of suffering influenced a generation of humanistic physicians. This publication provides a fuller treatment of the issues he raised in his 1982 article on “The Nature of Suffering.”
E. Cassell, The Nature of Suffering and the Goals of Medicine (New York: Oxford University Press, 1991).
The Nature of Suffering and the Goals of Medicine
Professionalism and Ethics
Publication of David Rothman’s Strangers at the Bedside: A History of How Law and Bioethics Transformed Medical Decision Making
Rothman’s social history of the transformation of bedside medical decision-making from the sole purview of the medical profession to new scrutiny of philosophers, theologians and lawyers begins with Henry Beecher’s 1966 New England Journal of Medicine article exposing abuses of research and the controversies that arose from the allocation of scarce dialysis and organ transplant resources and subsequent technological discoveries and consequent ethical challenges, including the issue of limitation of life-sustaining medical treatment exemplified in the Karen Ann Quinlan case (1976).
D. Rothman, Strangers at the Bedside: A History of How Law and Bioethics Transformed Medical Decision Making(New York: Basic Books, 1991).
Strangers at the Bedside: A History of How Law and Bioethics Transformed Medical Decision Making
Medical Aid in Dying
End-of-Life Issues
Clinician-Patient Relationship
Publication of Derek Humphry’s Final Exit: The Practicalities of Self-Deliverance and Assisted Suicide for the Dying
Humphry, co-founder of the Hemlock Society and Final Exit Network, published a best-selling how-to manual of techniques in nonmedical assistance in suicide for the dying, along with descriptions of applicable laws.
D. Humphry, Final Exit: The Practicalities of Self-Deliverance and Assisted Suicide for the Dying (New York: Dell Publishing, 1991).
Final Exit: The Practicalities of Self-Deliverance and Assisted Suicide for the Dying
Arts and Ethics
Public Health Ethics
Sexual and Gender Ethics
Angels in America: A Gay Fantasia on National Themes. A Play by Tony Kushner

Pulitzer Prize and Tony Award winning drama in two parts that portrays the evolution of the HIV/AIDS crisis in the U.S., including ethical issues of confidentiality and disclosure, stigma and abandonment, and access to promising clinical trials, through the eyes of individuals in the gay community, including prominent lawyer Roy Cohn, using fantasy and metaphor.
Angels in America: A Gay Fantasia on National Themes
Arts and Ethics
Professionalism and Ethics
The Doctor. Film from Touchstone Pictures
Based on the book A Taste of My Own Medicine by physician-writer Edward Rosenbaum, the film depicts an arrogant and distant surgeon whose professional and personal life and perspectives are changed when he is diagnosed with laryngeal cancer and undergoes treatment, during which he connects with the experience of other patients, including one who teaches him important lessons as she is dying. When his cancer is cured and he is fully recovered, he transforms into a model of empathy and compassion in his care of patients and teaching.
Bioethics Organizations
International Bioethics
Founding of Nuffield Council of Bioethics
The Nuffield Council of Bioethics is an independent body based in Great Britain. Established by the Trustees of the Nuffield Foundation, and later funded in conjunction with Wellcome and the Medical Research Council, it “examines and reports on ethical issues in biology and medicine,” and advises policy makers.
Journals in Bioethics
Publication of the Kennedy Institute of Ethics Journal
An interdisciplinary quarterly journal of the Joseph and Rose Kennedy Institute of Ethics (Georgetown University, Washington, D.C.) that publishes philosophically rigorous and empirically informed articles in all areas of bioethics (broadly construed) and on related issues in practical ethics.
Kennedy Institute of Ethics Journal
1992
Journals in Bioethics
Publication of the Cambridge Quarterly of Healthcare Ethics
Explores the many implications of both the broader issues in health care and society, and of organizational concerns arising in the institutions in which ethics committees are located.
Cambridge Quarterly of Healthcare Ethics
Organizational Ethics
Joint Commission Establishes Requirement of a Mechanism to Address Ethical Issues in Health Care
The Joint Commission on Accreditation of Healthcare Organizations (JCAHO) first required a “mechanism(s) for the consideration of ethical issues in the care of patients and to provide education to caregivers and patients on ethical issues in health care” in its Accreditation Manual. Though the standard does not require ethics committees per se, and ethics committees were noted (though the description was at best incomplete) in the Karen Ann Quinlan case as a potential aid in the resolution of difficult ethical cases, this standard fostered the proliferation of ethics committees in hospitals. At the time of Quinlan, ethics committees were rare; today they are nearly ubiquitous.
Joint Commission on Accreditation of Healthcare Organizations, Accreditation Guide for Hospitals, Oak Park, IL: JCAHO, 1992.
End-of-Life Issues
Clinician-Patient Relationship
Publication of Alan Meisel’s Article, “The Legal Consensus about Forgoing Life-Sustaining Treatment: Its Status and Its Prospects”
This landmark article after the Cruzan case summarized the legal consensus in the United States about foregoing life-sustaining medical treatment, a consensus that still remains today, namely that: 1) Individuals have the right to refuse any intervention, including ventilators, feeding tubes, blood products (see Bartling, Cal.App. 1984, Bouvia, Cal.App. 1986, Wons, Fla. 1989, Fosmire, N.Y.1990); 2) All patients have the right, even incapacitated (e.g. Quinlan,N.J. 1976, Cruzan, U.S. 1990); 3) Withholding and withdrawing medical treatment are not homicide or suicide, even if the patient is not in a terminal condition (e.g. Barber, Cal.App. 1983, Cruzan, U.S. 1990); orders to withhold treatment are valid (e.g. Dinnerstein, Mass. 1978); and 4) Courts need not be involved in these cases, now that the precedents have been set. This article has delineated for bioethicists, attorneys, and judges the general legal parameters of withholding and withdrawing medical treatment while assuaging clinicians, patients, and families that such ethical actions are also legal.
A. Meisel, “The Legal Consensus About Forgoing Life-Sustaining Treatment: Its Status and Its Prospects,” Kennedy Institute of Ethics Journal 2, no. 4 (1992): 309-45.
The Legal Consensus About Forgoing Life-Sustaining Treatment: Its Status and Its Prospects
Professionalism and Ethics
Publication of Howard Brody’s The Healer’s Power
Using literary examples including Dostoevsky and William Carlos Williams, Howard Brody examines the power inherent in the doctor-patient relationship, and its power to save lives and to heal, as well as the abuses of its power, including paternalistic and financial conflicts, and conflicts from the health care system. Rather than diminishing the healer’s power, instead the power should be tempered and used to share decision-making with patients, and to advocate for patients individually and collectively through health care resource allocation.
H. Brody, The Healer’s Power (New Haven: Yale University, 1992).
Reproductive Ethics
Publication of Bonnie Steinbock’s Life before Birth: The Moral and Legal Status of Embryos and Fetuses
Philosopher Bonnie Steinbock examines prenatal dilemmas based on analyses of interests and their moral consequences as applied to embryos and fetuses, the implications of consciousness and nonconsciousness, as well as the treatment of these issues in tort and criminal law.
B. Steinbock, Life before Birth: The Moral and Legal Status of Embryos and Fetuses(New York: Oxford University Press, 1992).
Life before Birth: The Moral and Legal Status of Embryos and Fetuses
Bioethics Organizations
International Bioethics
Founding of International Association of Bioethics (IAB)
Founded by Peter Singer, Dan Wikler, and Helga Kuhse to link those working in bioethics and related fields internationally and to encourage the discussion of cross-cultural issues in bioethics. The International Association of Bioethics held its First World Congress in Amsterdam and continues its mission through its work and its biennial congresses held around varied world venues.
The International Association of Bioethics
Bioethics Organizations
Feminist Bioethics
Founding of International Network on Feminist Approaches to Bioethics (FAB)
Launched at the First World Congress of the International Association of Bioethics (IAB), feminist scholars, led by philosopher Anne Donchin and biologist Helen (Becky) Holmes, created an organization to “encompass standpoints of women and other marginalized social groups” and called for new methodologies and strategies responsive to the disparate conditions of women’s lives around the globe.” FAB holds world congresses most commonly in conjunction with IAB.
1993
Bioethics Organizations
International Bioethics
UNESCO International Bioethics Committee
Created in 1993, the United Nations Educational, Scientific and Cultural Organization’s (UNESCO) International Bioethics Committee (IBC) is “a body of 36 independent experts that follows progress in the life sciences and its applications in order to ensure respect for human dignity and freedom.” The committee provides recommendations that are submitted to the Director-General, who disseminates them to UN Member States, the Executive Board, and the General Conference. The IBC has produced a Universal Declaration on Bioethics and Human Rights and has made recommendations on ethical issues in a wide range of topics, including the use of genomic data and response to the Ebola epidemic.
UNESCO International Bioethics Committee
International Bioethics
End-of-Life Issues
Clinician-Patient Relationship
Rodriguez, Supreme Court of Canada
A 42-year-old woman with amyotrophic lateral sclerosis (ALS) challenged the section of the Criminal Code that made assistance in suicide illegal, even if performed by a physician for a terminal patient. The court found that there was no right to assistance in dying for terminal patients in the Canadian Charter of Rights and Freedoms, a position it would later reverse in Carter v. Canada (2015), where it would find that the criminal code violated three provisions of the Charter concerning right to life, liberty, and security of the person.
Rodriguez v. British Columbia (AG)[1993] 3 SCR 519, 107 DLR (4th) 342, 1993 CanLII 75 – A
Rodriguez, Supreme Court of Canada
Professionalism and Ethics
Gold Foundation Begins White Coat Ceremony for Matriculating Medical Students
The first “White Coat Ceremony,” where new medical students are clothed by mentors in their white coats and recite a common pledge to emphasize the humanistic mission of medicine, was instituted at the Columbia University College of Physicians & Surgeons. Led by Arnold P. Gold, MD, Professor of Clinical Neurology and Professor of Clinical Pediatrics, and the foundation he founded, the ceremony provides an early counterpart to the Hippocratic Oath the students would take at graduation almost four years later. With support from the Gold and Robert Wood Johnson Foundations, the ceremony would be adopted across the U.S. and Canada. The Gold Foundation also instituted the Gold Humanism Honor Society chapters at most U.S. medical schools, as well as other recognitions, such as the Leonard Tow Humanism Award for faculty and students at each school, as well as other chapter activities, to advance humanism in medicine. The Gold Humanism Honor Society students are exemplars of compassionate patient care who serve as role models, mentors, and leaders in medicine; GHHS members are peer nominated and are the ones that others say they want to care for their own family.
The Gold Foundation White Coat Ceremony
Ethical Theory and Methods
Publication of Edmund Pellegrino and David Thomasma’s The Virtues in Medical Practice
Pellegrino and Thomasma revivify virtue theory in bioethics and its application in clinical ethics. Along with Alasdair MacIntyre’s After Virtue, published in 1981, Pellegrino and Thomasma would influence philosophers, theologians, and clinicians who found in virtue theory the basis for the moral core of medicine, the importance of virtue and character in its practitioners, and the foundations for resolving modern clinical ethical dilemmas.
E. Pellegrino and D Thomasma, The Virtues in Medical Practice(New York: Oxford University Press, 1992).
The Virtues in Medical Practice
Reproductive Ethics
End-of-Life Issues
Clinician-Patient Relationship
Publication of Ronald Dworkin’s Life’s Dominion: An Argument about Abortion, Euthanasia, and Individual Freedom
Dworkin analyzes the philosophical, historical, legal, medical, and theological perspectives on these vexing issues at the beginning and end of life, arguing that these decisions should be individual ones. The book’s broad and deep analysis had a major impact on further analyses of these issues by the bioethics community.
R. Dworkin’s Life’s Dominion: An Argument about Abortion, Euthanasia, and Individual Freedom(New York: Alfred A. Knopf, 1993).
Life’s Dominion: An Argument about Abortion, Euthanasia, and Individual Freedom
International Ethics
End-of-Life Issues
Clinician-Patient Relationship
Airedale NHS Trust vs Tony Bland, House of Lords, United Kingdom
The U.K. House of Lords authorized the removal of the feeding tube from Anthony (Tony) Bland, who had been in a persistent vegetative state since being crushed by a crowd during a football match in Hillsborough in 1989. Parents and physicians agreed to terminate life-prolonging medical treatment in the form of artificial nutrition and hydration. The hospital successfully applied for a court order allowing the withdrawal of artificial nutrition and hydration. This was the first English patient to be allowed by the court to die by this means.
Airedale NHS Trust vs Tony Bland (1993) 1 All ER 821 HL
Journals in Bioethics
Publication Commences for The Journal of Law, Medicine & Ethics
Then renamed from Law, Medicine & Health Care (itself having been formed from a combination of Medicolegal News and Nursing Law & Ethics in 1981). Published by Sage in partnership with the American Society of Law, Medicine, and Ethics, it is a quarterly peer-reviewed journal addressing medical ethics and medical law
The Journal of Law, Medicine & Ethics
1994
Bioethics Commissions and Other Government Entities
Research Ethics
Advisory Committee on Human Radiation Experiments
The Advisory Committee on Human Radiation Experiments (ACHRE) was created in 1994 by President Clinton to investigate and report on the use of federally funded research using ionizing radiation on human subjects. Ruth Faden, PhD, MPH, led the committee in its ethical review of experiments conducted in conjunction with atmospheric atomic testing, intentional releases, and other population exposures. The committee made findings about the human radiation experiments: their number and purpose, the likelihood that they produced harm, and how human radiation experimentation contributed to advances in medicine, as well as the ethics and regulations of the era, and made recommendations for actions that must be taken to ensure that the ends of national security and the advancement of medicine will “proceed only through means that safeguard the dignity, health, and safety of the individuals and groups who may be put at risk in the process.” These included changes in the current federal system for the protection of the rights and interests of human subjects through “changes in institutional review boards; in the interpretation of ethics rules and policies; in the conduct of research involving military personnel as subjects; in oversight, accountability, and sanctions for ethics violations; and in compensation for research injuries.” The report adds to the legacy of retrospective reviews of research such as the 1972 panel review of the Tuskegee research by the U.S. Assistant Secretary for Health and Scientific Affairs, among others.
Advisory Committee on Human Radiation Experiments (1994-95); report (1995)
The Advisory Committee on Human Radiation Experiments
Advisory Committee on Human Radiation Experiments Final Report
Medical Aid in Dying
End-of-Life Issues
Clinician-Patient Relationship
Oregon Death with Dignity Act
Oregon Ballot Measure 16 (Death with Dignity) passed 51.3% (vs. 48.7%), after injunction for challenges, enacted in 1997 in Death with Dignity Act (ORS 127.800-995). The Death with Dignity Act was the first law allowing medical assistance in dying in the United States, restricting the procedure to competent patients with a terminal condition and a prognosis of less than 6 months, requiring a request in writing followed by a 15-day waiting period, with a 48-hour waiting period for the medications to be dispensed. Oregon’s 20-year experience with the procedure has shown that less than 4/1,000 patients who die avail themselves of the law, that the top reasons for use of the law include loss of autonomy (91%), loss of activities enjoyed (90%), loss of dignity (76%), loss of bodily functions (46%), and being a burden for family, friends, caregivers (44%); lesser reasons include pain (26%), with finances (4%) uncommonly a concern. Because of the residency requirement of six months before the law can be used by a patient, terminal patients rarely move to Oregon to use the law.
Bioethics Organizations
American Association for Bioethics (AAB)
The American Association for Bioethics (AAB), formed in the fall of 1994, “promoted the exchange of ideas among bioethics scholars, enhanced the clinical activities of bioethicists, encouraged discussion and research in bioethics and encouraged teaching and development of new scholars and participants in the field.” In 1998, the AAB merged with the Society for Bioethics Consultation (SBC) and the Society for Health and Human Values to form the American Society for Bioethics and Humanities (ASBH).
The American Society for Bioethics and Humanities History
Ethics Committees and Ethics Consultation
Publication of John La Puma and David Schiedermayer’s Ethics Consultation: A Practical Guide
Internists and trained clinical ethicists John La Puma and David Schiedermayer, published their step-by-step guide to clinical ethics consultation by a solo consultant along the model of medical consultation. The volume created some controversy in its claim that ethics consultation is best done by clinicians, i.e., those trained in clinical medicine or nursing, with additional ethics training. Many respondents argued that training in clinical areas was not essential to bedside clinical ethics consultation, and La Puma and Schiedermayer would later expand their analysis of what constituted the clinical knowledge, training, and experience that might allow nonclinicians to have the clinical savoir faire to participate in bedside consultation.
J. LaPuma and D. L. Schiedermayer, “Ethics Consultation: A Practical Guide,” HEC Forum 6, no. 3 (1994): 163-69.
Ethics consultation: a practical guide
Reproductive Ethics
Publication of John Robertson’s Children of Choice: Freedom and the New Reproductive Technologies
Robertson sets forth a comprehensive theory of the evolving legal and ethical concept of procreative liberty, i.e., “the freedom to decide whether or not to reproduce and the freedom to reproduce when, with whom, and by what means one chooses.” He delineated the implications of procreative liberty, covering topics that include contraception, abortion, in vitro fertilization, surrogacy, genetic screening, conception for eventual organ donation, and creating embryos for research.
J. Robertson, Children of Choice: Freedom and the New Reproductive Technologies(Princeton, NJ: Princeton University Press, 1994).
Children of Choice: Freedom and the New Reproductive Technologies
Sexual and Gender Ethics
Publication of Timothy Murphy’s Ethics in an Epidemic: AIDS, Morality, and Culture
This early book on HIV/AIDS in bioethics discourse examines the epidemic’s challenge in a range and scope of questions of moral philosophy, at times overwhelming because of their magnitude.
T. Murphy. Ethics in an Epidemic: AIDS, Morality, and Culture (University of California Press, 1994)
Ethics in an Epidemic: AIDS, Morality, and Culture
Arts and Ethics
“ER” and “Chicago Hope” Television Series

A Peabody and Emmy (22) award-winning television series created by physician-writer Michael Crichton, “ER” ran for 15 seasons. Set in an urban teaching hospital, the series followed clinician and patient stories that included scores of medical ethics issues. As one of the most watched television series, it brought to public attention many ethical dilemmas faced by clinicians. Another Emmy award winning (7) series that began the same year, “Chicago Hope,” ran for 6 seasons, and also incorporated ethical issues. “ER” and “Chicago Hope” may have contributed to an unrealistic expectation by the public of the likelihood of success of cardiopulmonary resuscitation (CPR), since most patients on TV were successfully resuscitated (without neurological deficits), while most patients actually are not successfully resuscitated.
Diem, S. J., Lantos, J. D., Tulsky, J. A. “Cardiopulmonary Resuscitation on Television — Miracles and Misinformation,” New England Journal of Medicine 334 (1996):1578-82.
Cardiopulmonary Resuscitation on Television — Miracles and Misinformation
https://www.tvguide.com/tvshows/chicago-hope/100084/
1995
End-of-Life Issues
Clinician-Patient Relationship
The SUPPORT Trial on End-of-Life Care & Dying Well in the Hospital: The Lessons of SUPPORT
A two-year prospective observational study (phase I) with 4301 patients followed by a two-year controlled clinical trial (phase II) with 4804 patients and their physicians randomized to the intervention group or control group in five teaching hospitals in the United States that showed substantial shortcomings in care for seriously ill hospitalized adults (phase I) and failure of intervention to improve care or patient outcomes (phase II). This was followed by a foundational document on end-of-life care practices, 12 commenters discuss the implications of the SUPPORT study.
The SUPPORT Principal Investigators. “A Controlled Trial to Improve Care for Seriously Ill Hospitalized Patients. The Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT),” Journal of the American Medical Association 274, no. 20 (1995): 1591-98.
E. H. Moskowitz and J. Lindemann, “Dying Well in the Hospital: The Lessons of SUPPORT,”special report, Hastings Center Report 25, no. 6 (1995): S2-S36.
Dying Well in the Hospital: The Lessons of SUPPORT
Professionalism and Ethics
Publication of Bernard Lo’s Resolving Ethical Dilemmas: A Guide for Clinicians
Internist and ethicist Bernard Lo created a clinician-friendly textbook with cases and analyses for use in medical and postgraduate medical education that has been adopted by many medical schools.
B. Lo, Resolving Ethical Dilemmas: A Guide for Clinicians (Baltimore: Williams & Wilkins, 1995).
Resolving Ethical Dilemmas: A Guide for Clinicians
Arts and Ethics
Public Health Ethics
Outbreak. A Film from Warner Bros. Studios.

Based on the book The Hot Zone by Richard Preston, the film is a plague narrative that depicts an outbreak in Africa of a highly infectious airborne virus. The movie brought to a wide audience the public health ethical issues that might arise from an Ebola-like virus.
1996
Privacy and Confidentiality
Health Insurance Portability and Accountability Act of 1996 (HIPAA)
The passage of HIPAA serves several functions, including protecting health insurance coverage for workers and their families when they change or lose their jobs, and establishing the first national standard to protect patients’ personal or protected health information (PHI) in electronic, written, or oral form by limiting its use and disclosure (HIPAA Privacy Rule). HIPAA helps promote a culture of respect for patient privacy and confidentiality in the handling of their PHI.
Health Insurance Portability and Accountability Act of 1996, Pub. L. No. 104-191, 110 Stat. 1936 (1996).
Health & Human Services: HIPPA
HIPPA Combined Regulation Text
Cloning
Genomics and Ethics
Birth of Dolly

Dolly is cloned by researchers at the Roslin Institute in Scotland from an adult cell taken from the mammary gland of a 6-year-old Finn Dorset sheep and an egg cell taken from a Scottish Blackface sheep. Dolly’s birth demonstrates that specialized cells can be used to create an exact copy of the animal they came from. The birth of Dolly creates an awareness that ordinary cells can be reprogrammed to become pluripotent stem cells that can be grown into any tissue. Thus, Dolly led to advances in stem cell research.
Resource Allocation
Publication of Norman Daniels’ Justice and Justification: Reflective Equilibrium in Theory and Practice
In this collection of essays Daniels presents a wide approach to reflective equilibrium (or coherence) with implications for future theoretical work and practical applications. Some of the addressed issues include the design of health care institutions and the fair distribution of goods between the old and the young.
N. Daniels, Justice and Justification: Reflective Equilibrium in Theory and Practice (New York: Cambridge University Press, 1996)
Justice and Justification: Reflective Equilibrium in Theory and Practice
Animal Rights
Publication of David DeGrazia’s Taking Animals Seriously: Mental Life and Moral Status
DeGrazia discusses whether equal consideration should be extended to animals’ interests, and critically examines the issues of animals’ minds and well-being.
D. DeGrazia, Taking Animals Seriously: Mental Life and Moral Status (New York: Cambridge University Press)
Taking Animals Seriously: Mental Life and Moral Status
Feminist Ethics
Publication of Susan Wolf’s Feminism and Bioethics: Beyond Reproduction
This volume of original essays moves beyond the traditional bioethics topics of reproduction and nursing. The book starts with an investigation of the relationship between feminism and bioethics and introduces different approaches, such as liberal feminism, to a variety of contemporary issues, including problems, such as euthanasia, the definition of health, healthcare-patient communication, and the conduct of biomedical research.
S. Wolf, Feminism and Bioethics: Beyond Reproduction (Oxford University Press)
Feminism and Bioethics: Beyond Reproduction
Bioethics Commissions and Other Government Entities
Research Ethics
Genomics and Ethics
National Bioethics Advisory Commission (NBAC)

The National Bioethics Advisory Commission (1996-2001) is established to advise, consult with, and make recommendations to the National Science and Technology Council, federal agencies, and other appropriate entities, and also provide the public with advice and recommendations. NBAC is charged with identifying broad, overarching principles to govern the ethical conduct of research. President Clinton’s Executive Order (12975 on October 3, 1995) establishing NBAC assigned a two-fold priority: the protection of the rights and welfare of human research subjects; and issues in the management and use of genetic information, including but not limited to, human gene patenting.
National Bioethics Advisory Council: General Information
Presidential Order Establishing NBAC
1997
Medical Aid in Dying
End-of-Life Issues
Clinician-Patient Relationship
U.S. Supreme Court Case—Vacco v. Quill
In Vacco v. Quill, Dr. Timothy Quill and other physicians and three seriously ill patients challenge the constitutionality of New York State’s ban on physician-assisted suicide. The Supreme Court holds that the U.S. Constitution does not guarantee the right to die and that physician-assisted suicide is not a constitutionally protected interest under the Equal Protection Clause of the 14th Amendment. The Court affirms that New York’s ban on physician-assisted suicide is rationally related to a legitimate state interest. The Court also concludes that there is a legal distinction between letting a patient die and making that patient die.
Vacco v. Quill, 521 U.S. 793 (1997).
https://supreme.justia.com/cases/federal/us/521/793/
Medical Aid in Dying
End-of-Life Issues
U.S. Supreme Court Case-Washington v. Glucksberg
In the Washington v. Glucksberg case, Dr. Harold Glucksberg with other physicians, three terminally ill patients, and a nonprofit organization that counseled individuals contemplating physician-assisted suicide challenge Washington State’s ban on physician-assisted suicide. Glucksberg argues that Washington’s ban on physician-assisted suicide violates the Fourteenth Amendment’s Due Process Clause by denying mentally competent, terminally ill adults the liberty to choose death over life. The Supreme Court holds that Washington’s prohibition against causing or aiding a suicide does not violate the Due Process Clause.
Washington v. Glucksberg, 521 U.S. 702 (1997).
https://supreme.justia.com/cases/federal/us/521/702/case.pdf/
Medical Aid in Dying
End-of-Life Issues
Oregon’s Death with Dignity Act is Implemented

Oregon is the first state to pass a law allowing physicians to prescribe medications to be self-administered by terminally ill patients to hasten their death (sometimes referred to as physician-assisted dying, medical aid-in-dying, and physician-assisted suicide). The implementation of this Act (which was passed in 1994) goes into effect after Oregonians defeated a ballot measure in 1997 aimed at repealing the law. The Act withstands multiple attempts to nullify it, both legislatively, in the Oregon legislature and the U.S. Congress, and in federal courts. In 2006, the U.S. Supreme Court rules that Oregon physicians can prescribe life-ending medication under the Act. Over time, the Oregon experience shows that death with dignity accounts for a very small percentage of deaths. In addition, concerns about physician-assisted dying being forced on vulnerable adults is unsupported by the data.
Oregon’s Death with Dignity Act
Oregon’s Death with Dignity Act History
Oregon’s Death with Dignity Act: 20 Years of Experience Inform Debate
Research Ethics
International Ethics
Publication of Peter Lurie and Sidney Wolfe’s “Unethical Trials of Interventions to Reduce Neonatal Transmission of Human Immunodeficiency Virus in Developing Countries”
Peter Lurie and Sidney Wolfe report that after research demonstrates reduced HIV transmission to newborn infants, the standard of care for HIV-positive pregnant women becomes the regimen used in the AIDS Clinical Trials Group (ACTG) Study 076. However, in developing countries, the potential cost of the ACTG regimen prevents access. Follow-up research studies in developing countries often do not provide patients with antiretroviral drugs or use placebos in the control groups. The article highlights that researchers often ask the wrong question by not assessing the effectiveness of less expensive interventions, do not appreciate the ethically challenged use of placebos, and fail to appreciate the implications of using a standard of care that does not conform to the standard of care in the sponsoring country.
P. Lurie and S. Wolfe, “Unethical Trials of Interventions to Reduce Neonatal Transmission of Human Immunodeficiency Virus in Developing Countries,” New England Journal of Medicine 337, no. 12 (1997): 853-56.
Ethical Theory and Methods
Publication of Bernard Gert, Charles Culver, and K. Danner Clouser’s Bioethics—A Return to Fundamentals
This book emphasizes the concept of a common morality, and explores and clarifies the relationship between common morality and bioethics. Core concepts in bioethics are explicated and grounded in common morality.
B. Gert, C. Culver, and K. Danner Clouser, Bioethics—A Return to Fundamentals(New York: Oxford University Press, 1997).
Bioethics—A Return to Fundamentals
Narrative Ethics
Publication of Anne Fadiman’s The Spirit Catches You and You Fall Down: A Hmong Child, Her American Doctors, and the Collision of Two Cultures
Anne Fadiman portrays a Hmong refugee family from Laos as they grapple with the health care system in California.
A. Fadiman, The Spirit Catches You and You Fall Down: A Hmong Child, Her American Doctors, and the Collision of Two Cultures (New York: Farrar, Straus and Giroux, 1997).
Research Ethics
Genomics and Ethics
Reproductive Ethics
Cloning Human Beings
The first report of the recently appointed National Bioethics Advisory Commission (NBAC) focuses on the ethical and public policy issues raised by the prospect of human reproductive cloning in the light of the announcement of Dolly’s birth. The report, which was requested by President Clinton, with a 90-day deadline, calls for a federal ban on attempts to create a child through somatic cell nuclear transfer cloning (with a sunset clause of 3 to 5 years). The report found such attempts to be “morally unacceptable” at the time on grounds of safety and the need for “widespread and careful public deliberation” on “many other serious ethical concerns.”
Cloning Human Beings: Report and Recommendations of the National Bioethics Advisory Commission (Rockville, MD: NBAC, 1997)
Cloning Human Beings: Report and Recommendations of the National Bioethics Advisory Commission
1998
Ethics of Enhancement
Technology
Conclusions of Research “On the Prospect of Technologies Aimed at the Enhancement of Human Capacities and Traits”
This project has served as an important anchor for the subsequent discussion of a wide variety of technologies. The technologies have extended from cosmetic surgery to brain-computer interfaces.
E. Parens, ed., Enhancing Human Traits: Ethical and Social Implications (Washington, DC: Georgetown University Press, 1998).
E. Parens, “Is Better Always Good?” Hastings Center Report 28, no. 1 (1998). https://onlinelibrary.wiley.com/doi/full/10.2307/3527981
Bioethics Organizations
ASBH, SHHV, and AAB Merger
The American Society for Bioethics and Humanities (ASBH) is founded through the consolidation of three existing associations in the field: Society for Health and Human Values (SHHV), Society for Bioethics Consultation (SBC), and American Association of Bioethics (AAB). The Society for Health and Human Values (SHHV) was a professional membership organization that promoted informed concern for human values in the education of health professionals. SBC was the first specialty group to focus on ethics consultation. AAB promoted the exchange of ideas among bioethics scholars, enhanced the clinical activities of bioethicists, encouraged discussion and research in bioethics, and encouraged teaching and development of new scholars and participants in the field. ASBH conducts annual conferences, produces publications to develop competencies in health care ethics, and produces guidelines, such as the Code of Ethics and Professional Responsibilities for Healthcare Ethics Consultants.
Medical Aid in Dying
End-of-Life Issues
Clinician-Patient Relationship
Dr. Jack Kevorkian Charged
Dr. Jack Kevorkian, a trained pathologist, started providing death counseling in the late 1980s. His first public assisted suicide occurred in 1990 and involved Janet Adkins, a 54-year-old woman with Alzheimer’s disease. Kevorkian reportedly assists in the deaths of more than 100 terminally ill people. In each of these cases, the individuals themselves allegedly take the final action which resulted in their own deaths. Kevorkian is tried four times for assisting suicides without a conviction. In the November 22, 1998 broadcast of CBS News (60 Minutes) Kevorkian allows the airing of a videotape which depicts him voluntarily euthanizing Thomas Youk, a 52-year-old man with end-stage Lou Gehrig’s disease. In this case, Kevorkian administered the lethal injection. As a result, he is charged with second-degree murder and possessing/delivering a controlled substance. He is found guilty and is sentenced to serve 10 to 25 years in prison.
Dr Kevorkian found guilty of second degree murder
Why ‘Dr. Death’ Wanted to Be Charged with Murder
Genomics and Ethics
Deriving and Maintaining Human Embryonic Stem Cells
Dr. James Thomson and colleagues publish a paradigm shifting article in Science in which they report deriving human embryonic stem cells (ESCs) used in experimentation from donated embryos originally produced for in vitro fertilization. After informed consent and institutional review board approval, the human embryos are cultured and supported so that cells have the developmental potential to form derivatives of all three embryonic germ layers (endoderm, mesoderm, and ectoderm). These cell lines are thought to have great potential to be useful in human developmental biology, drug discovery, and transplantation medicine. Thompson’s experimentation paves the way for future experiments utilizing human ESCs.
Embryonic Stem Cell Lines Derived from Human Blastocysts
Man Who Helped Start Stem Cell War May End It
Genomics and Ethics
Celera Genomics Founded

J. Craig Venter founded Celera Genomics in 1998 and with private funding pursued sequencing the human genome. Francis Sellers Collins, the head of the publicly funded Human Genome Project, was pursuing the same goal since the early 1990s. They joined forces after President Bill Clinton and British Prime Minister Tony Blair made a joint declaration in 2000 that all genome information should be free to the public. This announcement led to cooperation between Collins and Venter, and on June 26, 2000, Venter and Collins jointly announced that, after nearly a decade of work, both the public Human Genome Project headed by Collins and Celera Genomics headed by Venter had deciphered essentially all the genes in human DNA. Their reports appeared in 2001.
Sequencing Human Genome: the Contributions of Francis Collins and Craig Venter
The Sequence of the Human Genome
Initial sequencing and analysis of the human genome
Journals in Bioethics
First Publication of the American Journal of Bioethics
The American Journal of Bioethics (AJOB) is a monthly peer-reviewed academic journal published by Taylor & Francis, covering all aspects of bioethics. AJOB presents target articles, open peer commentaries, editorials, book reviews, case studies, and commentaries in clinical and research ethics. AJOB also produces independently managed journals on neuroscience and empirical bioethics as spin-offs.
The American Journal of Bioethics, Current Issue
End-of-Life Issues
Professionalism and Ethics
Clinician-Patient Relationship
The Initiative for Pediatric Palliative Care
This decades-long education and research initiative conducted by the Education Development Center in Newton, MA was led by an interdisciplinary team of educators, clinicians, and social scientists from major academic medical centers. Trainings, based on the research findings and novel educational materials, were delivered to teams that came from nearly all children’s hospitals in the United States. The curriculum and training workshops addressed barriers to optimal palliative care for children, such as reluctance to share decision-making with parents, confusion about futile care policies, reimbursement policies that unnecessarily made parents choose between cure or palliative care, and uncertainty about how much decisional authority adolescents should have. The 25-session curriculum was developed and is still free and available upon request.
M. Z. Solomon et al., “New and lingering controversies in pediatric end-of-life care,” Pediatrics 2005; 116: 872-883.
D. Browning and M.Z. Solomon, “The Initiative for Pediatric Palliative Care: An interdisciplinary educational approach for health care professionals,” Journal of Pediatric Nursing 2005; 20 (5): 326-34.
D. Browning and M.Z. Solomon, “Relational learning in pediatric palliative care: Transformative education and the culture of medicine.” Child and Adolescent Psychiatric Clinics of North America, 15: 795-815, 2006.
M.Z. Solomon et al., “Learning that leads to action: Impact and characteristics of a professional education approach to improve the care of critically ill children and their families.” Archives of Pediatric and Adolescent Medicine, 2010 April; 164(4): 315-22.
Initiative for Pediatric Palliative Care: Exemplar IPE Programs for Students
Ethical Theory and Methods
Publication of Carl Elliott’s A Philosophical Disease: Bioethics, Culture, and Identity
Elliott uses a Wittgensteinian approach to discuss issues about the moral challenges confronting modern medicine. Topics include illness and identity, psychopharmacology, the role of clinical ethicists, and narrative in medicine.
C. Elliott, A Philosophical Disease: Bioethics, Culture, and Identity(New York: Routledge, 1998)
A Philosophical Disease Bioethics, Culture, and Identity
Ethical Theory and Methods
Publication of Albert Jonsen’s The Birth of Bioethics

Jonsen examines the origin and evolution of the debates over human experimentation, genetic engineering, organ transplantation, termination of life-sustaining treatment, and new reproductive technologies. His book assesses the contributions of philosophy, theology, law, and the social sciences to the expanding discourse of bioethics.
A. Jonsen, The Birth of Bioethics (New York: Oxford University Press, 1998)
Bioethics Pedagogy
Initiation of Online Courses in Bioethics at the Medical College of Wisconsin Graduate Program (MA) in Bioethics
Medical College of Wisconsin initiates a program of online course in bioethics. The goal is to facilitate learning how to analyze and resolve difficult ethical issues that arise in health care institutions.
Medical College of Wisconsin Bioethics Programs and Certificates
1999
Disability Ethics
Conclusions and Publication of Research: “On Prenatal Testing and Disability Rights”
This project gave prominent attention to the social model of disability in general and to the disability-community critique of prenatal genetic testing in particular. It has served as an important anchor for the subsequent discussions of disability justice and reproductive justice.
E. Parens and A. Asch, “The Disability Rights Critique of Prenatal Genetic Testing: Reflections and Recommendations,” Hastings Center Report 29, no. 4 (1999): S1-S22. Hastings Center Special Report: Disability Rights Critique of Prenatal Genetic Testing
E. Parens and A. Asch. (Eds), Prenatal Testing and Disability Rights, Washington, DC: Georgetown University Press, 2000, Prenatal Testing and Disability Rights on Amazon
Research Ethics
Genomics and Ethics
Case of Jesse Gelsinger
Jesse Gelsinger, age 18, develops respiratory failure and dies after receiving experimental new gene therapy. In a wrongful death lawsuit against the researchers, the University of Pennsylvania and Children’s Hospital of Philadelphia, numerous problems are identified. The Gelsinger case leads to efforts to promote ethical conduct of clinical research and to better protect patient safety in trials. The case highlights the uncertainty of genotoxicity as a risk that needs to be communicated in the informed consent process and the possible introduction of bias in presenting information about a research trial when an investigator or institution has a conflict of interest.
NY Times: The Biotech Death of Jesse Gelsinger
Nature: Gene-therapy trials must proceed with caution
Journals in Bioethics
Publication of the AMA Journal of Ethics
The AMA Journal of Ethics was established as Virtual Mentor but returns to its current name in 2015. This journal helps students, residents, and physicians explore and address the ethical challenges emerging from new medical technologies, changing patient expectations, and shifting public priorities. Commentaries and review articles offer practical guidance and foster professional reflection on timely ethical issues in health care. Each monthly issue of the AMA Journal of Ethics focuses on a theme selected by a medical student or resident physician who serves as issue editor.
2000
Research Ethics
NIH and OHRP-Training in Research Ethics
The National Institutes of Health (NIH) and the Office for Human Research Protections (OHRP), situated within the U.S. Department of Health and Human Services (HHS), require all people conducting or overseeing human subjects research have some training in research ethics. OHRP provides guidance for the protection of the rights, welfare, and well-being of human subjects involved in research conducted or supported by the HHS. OHRP oversees and ensures compliance with a set of regulations for Institutional Review Boards (IRB) that cover human test subjects in research, including those pertaining to the “Common Rule” (see Publication of the Common Rule in the 1991 timeline entry). OHRP’s regulatory compliance activities include suspension of research experiments when violations are identified. Within OHRP, the Division of Education and Development (DED) provides education for IRBs, gives guidance on research ethics, and advises the HHS Secretary on issues of medical ethics. The educational section of the OHRP website supports the IRB and research communities in their efforts to protect human subjects in research. Much of DED’s educational materials are for public use and distribution.
US Department of Health & Human Services
OHRP suspends Johns Hopkins Research license for Fed funded research
Genomics and Ethics
Publication of Allen Buchanan, Daniel Brock, Norman Daniels, and Daniel Wikler’s From Chance to Choice: Genetics and Justice
This book addresses the ethical issues underlying the application of genetic technologies to human beings. Probing the implications of the remarkable advances in genetics, the authors ask how these should affect our understanding of distributive justice, equality of opportunity, the rights and obligations of parents, the meaning of disability, and the role of the concept of human nature in ethical theory and practice.
A. Buchanan et al., From Chance to Choice: Genetics and Justice (New York: Cambridge University Press, 2000).
From Chance to Choice: Genetics and Justice on Amazon
2001
Research Ethics
Genomics and Ethics
President George W. Bush’s Ban on Funding for Research on Human Embryonic Stem Cell Lines
President Bush presents a policy on federal funding for research on newly created human embryonic stem cells (ESC). President Bush acknowledges the importance of issues surrounding ESC research, presents different arguments in favor of and against ESC research, and explains his decision to limit but not completely eliminate potential federal funding for ESC research. More specifically, the policy specifies that federal funds can only be used for research on existing stem cell lines that are derived with the informed consent of the donors, from excess embryos created solely for reproductive purposes, and without any financial inducements to the donors. The policy specifies that research on lines created prior to that date continue to be eligible for funding. As a result, seventy-one cell lines meet the eligibility criteria, and researchers who wish to investigate these lines can still receive grants through the National Institutes of Health. However, only 21 stem cell lines are available and viable for research purposes. In the same speech, Bush establishes a registry of stem cell lines and announces the creation of a special council to oversee stem cell research. The ban against using federal funds for ESC research forces some researchers to create dichotomous research environments; one based on federal funding and another based on private funding. The ban also impacts international collaboration and communication.
President George W. Bush’s Announcement on Stem Cells, 9 August 2001
Ethical Theory and Methods
Publication of Jeremy Sugarman and Daniel Sulmasy’s Methods in Medical Ethics
Jeremy Sugarman and Daniel Sulmasy publish an edited volume that systematically examines methods from various disciplines, including history, philosophy, law, economics, and various approaches including qualitative, quantitative and experimental methods that are pursued by researchers in medical ethics. The second edition (2010) updates the materials significantly.
J. Sugarman and D. Sulmasy, Methods in Medical Ethics (Washington, D.C.: Georgetown University Press, 2001).
Methods in Medical Ethics on Georgetown Press
Bioethics Commissions and Other Government Entities
President’s Council on Bioethics
In late 2001, President George W. Bush establishes the President’s Council on Bioethics by Executive Order 13237 to “advise the President on bioethical issue that may emerge as a consequence of advances in biomedical science and technology.” Chaired first by Leon Kass and then by Edmund Pellegrino, the Council publishes numerous reports.
2002
Bioethics Commissions and Other Government Entities
Research Ethics
Publication of: Integrity in Scientific Research: Creating an Environment That Promotes Responsible Conduct
The Committee on Assessing Integrity in Research Environments, the National Research Council, and the Institute of Medicine develop a report that stresses the important role that research institutions play in providing an integrity-rich environment. Recommendations included the following:
- Funding agencies should establish research grant programs to identify, measure, and assess the factors that influence integrity in research.
- Research institution should (a) develop and implement a comprehensive program designed to promote integrity in research, (b) implement effective educational programs that enhance the responsible conduct of research, (c) evaluate and enhance the integrity of their research environments using a process of continuous quality improvement including self-assessment and external peer review, and (d) include institutional self-assessment of integrity in research as part of existing accreditation processes whenever possible.
- Office of Research Integrity (ORI) should establish and maintain a public database of institutions that are actively pursuing or employing institutional self-assessment and external peer-review of integrity in research.
Integrity in Scientific Research: Creating an Environment That Promotes Responsible Conduct
Integrity in Scientific Research: Creating an Environment That Promotes Responsible Conduct
Cloning
Genomics and Ethics
Bioethics Commissions and Other Government Entities
Publication of: Human Cloning and Human Dignity: An Ethical Inquiry
The President’s Council on Bioethics agrees that cloning to produce children is unsafe and morally unacceptable and ought not be attempted. Five areas of concern influence this opinion: problems of identity and individuality, concerns regarding manufacture, the prospect of a new eugenics, troubled family relations, and effects on society. Members of the Council did not come to agreement about cloning for biomedical research. Consequently, two policy options are presented: (1) a four-year moratorium on cloning for biomedical research with a federal review of current and projected practices of human embryo research, pre-implantation genetic diagnosis, genetic modification of human embryos and gametes, and related matters, with a view to recommending and shaping ethically sound policies for the entire field; and (2) regulation of the use of cloned embryos for biomedical research. This report influences President Bush to ban reproductive cloning and enact a moratorium on research cloning.
Human Cloning & Human Dignity: An Ethical Inquiry on Google Books
Bioethics Pedagogy
Initiation of Online Courses in Bioethics at the Neiswanger Institute for Bioethics (Loyola University Chicago)
The Neiswanger Institute for Bioethics implemented online courses in bioethics and emphasized two themes reflecting their institutional values. One commitment was to educate health care professionals to be effective leaders for social justice in medicine and society. The second theme was a commitment to promote understanding and respect for the role of religious and spiritual traditions, especially that of the school’s Roman Catholic heritage, in health care decision-making.
2003
Reproductive Ethics
Partial-Birth Abortion Ban Act
Partial-Birth Abortion Ban Act (2003) is a U.S. law that amends the federal criminal code to prohibit any physician or other individual from knowingly performing a partial-birth abortion, except when necessary to save the life of a mother whose life is endangered by a physical disorder, illness, or injury. Partial-birth abortion is often referred to in the medical literature as intact dilation and extraction. This Act defines a “partial-birth abortion” as an abortion in which the person performing the abortion: (a) deliberately and intentionally vaginally delivers a living fetus until, in the case of a head-first presentation, the entire fetal head is outside the mother’s body, or, in the case of a breech presentation, any part of the fetal trunk past the navel is outside the mother’s body; and (b) performs the overt act, other than completion of delivery, that kills the partially delivered living fetus. The Act prohibits the prosecution of a woman upon whom a partial-birth abortion is performed for conspiracy to violate this Act or under provisions regarding punishment as a principal or an accessory or for concealment of a felony.
Partial Birth Abortion Ban Act, 108-105, 117 Stat. 1201, enacted November 5, 2003, 18 U.S.C. § 1531
https://www.npr.org/2006/02/21/5168163/partial-birth-abortion-separating-fact-from-spin/
Social Justice
Publication of Brian Smedley, Adrienne Stith, and Alan Nelson’s Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care
Produced by the U.S. Institute of Medicine Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care.
B. Smedley, A. Stith, and A. Nelson, Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care (Washington, DC: National Academies Press, 2003).
‘Partial-Birth Abortion’: Separating Fact From Spin
Ethical Theory and Methods
Publication of Paul Farmer’s “Pathologies of Power: Health, Human Rights, and the New War on the Poor”
Farmer argues that promoting the social and economic rights of the world’s poor is the most important human rights struggle of our times. With eyewitness accounts, he links the lived experiences of individual victims to a broader analysis of structural violence, and exposes the relationships between political and economic injustice, on one hand, and the suffering and illness of the powerless, on the other.
P. Farmer, “Pathologies of Power: Health, Human Rights, and the New War on the Poor,” Journal for the Anthropology of North America 6, no. 1 (2003): 1-4.
Pathologies of Power: Health, Human Rights, and the New War on the Poor
2004
Organizational Ethics
Research Ethics
Publication of articles by F. Baylis and M. Rowell entitled, “The Olivieri Debacle: Where Were the Heroes of Bioethics?”
The articles in the Journal of Medical Ethics challenge the duty of bioethicists to speak truth to power. Dr. Nancy Olivieri, an expert in blood disorders, is testing the treatment of thalassemia major by using a medication called deferiprone. She comes to believe that this medication might be ineffective and toxic (probably causing liver fibrosis). She reports this to the drug sponsor of the research as well as to the Research Ethics Board at the hospital where she works. Her observations lead to proposed revisions in the informed patient consent form and drafting of a report to the Human Protection Branch of Health Canada. When the Canadian generic drug manufacturer learns of the proposed changes, they terminate the trial and inform Dr. Olivieri that all information about the trial is to remain confidential or there will be legal consequences. Despite this case presenting ethical challenges regarding research integrity, protection of research participants, informed consent, protection of the public interest, and academic freedom, the response from associated academic institutions is limited, in part due to conflicts of interest. Disappointingly, the response from associated bioethics programs and the larger bioethics community also is limited and often silent.
F. Baylis and M. Rowell, “The Olivieri Debacle: Where Were the Heroes of Bioethics?” Journal of Medical Ethics 30 (2004): 44-49.
The Olivieri debacle: where were the heroes of bioethics?
International Ethics
Research Ethics
Publication of Ruth Macklin’s Double Standards in Medical Research in Developing Countries
Macklin provides a policy-based argument that research populations in the developing world must, whenever possible, receive treatment and benefits equal to those enjoyed by their counterparts in the developed world. The book takes ethical guidelines that inform policy to focus on the practical application for the conduct of research.
R. Macklin, Double Standards in Medical Research in Developing Countries (New York: Cambridge University Press, 2004).

Double Standards in Medical Research in Developing Countries on Amazon
Genomics and Ethics
California Proposition 71 (California Stem Cell Research and Cares Act)
The Act establishes the sale of general obligation bonds for stem cell research and research facilities. Proposition 71 establishes the California Institute for Regenerative Medicine.
M. T. Longaker, L. C. Baker, H. T. Greely, “Proposition 71 and CIRM—Assessing the Return on Investment,” Nature Biotechnology 25, no. 5 (2007): 513-21.
Proposition 71 and CIRM—assessing the return on investment: 10.1038/nbt0507-513.
2005
End-of-Life Issues
Clinician-Patient Relationship
The Death of Terri Schiavo
Terri Schiavo had a cardiac arrest at age 26 and developed anoxic brain damage that resulted in a persistent vegetative state, requiring prolonged artificial life support, including a feeding tube. Her husband sought to remove the feeding tube because he felt that she would not have wanted prolonged artificial life support without the prospect of recovery. Terri’s parents disputed these assertions and challenged the diagnosis and their son-in-law’s decision-making authority as her guardian. The legal and political arguments pertaining to this case ultimately led President George W. Bush to sign legislation to move the case to the federal courts. Appeals to the federal court system upheld the decision to remove the feeding tube. This occurred and led to her death.
Bush v. Schiavo, 885 So. 2d 321, 336-37 (Fla. 2004)
An Act for the Relief of the Parents of Theresa Marie Schiavo, Pub. L. No. 109-3, 119 Stat. 15 (2005).
Schiavo ex rel. Schindler v. Schiavo, 358 F. Supp. 2d 1161, 1167 (M.D. Fla. 2005).
How Terri Schiavo Shaped the Right-to-Die Movement
Research Ethics
NIH and Conflict of Interest
Prior to 2005, NIH policies allow senior employees to consult and receive money, stock, and stock options in return for their advice. This is intended to facilitate recruitment of well-qualified investigators to conduct federally funded research. However, after media attention to possible conflicts of interest, Congress pressures the U.S. Department of Health and Human Services (the source of NIH budgets) to publish new regulations on conflicts of interest. The new rules prohibit all NIH employees (and in some instances, their spouses) from activities and investments such as receiving any compensation from the private sector or from research institutions that receive NIH grants, and from holding stock in biopharmaceutical companies. Although the reactions to the new rules are mixed and a few prominent NIH researchers leave the NIH for positions at major U.S. universities, open discussion is proposed to extend the conflict-of-interest rules to universities and other public institutions.
Department of Health and Human Services. Supplemental standards of ethical conduct and financial disclosure requirements for employees of the Department of Health and Human Services: interim final rule with request for comments.
Fed Reg 2005;70:5543-65
Bioethics Commissions and Other Government Entities
United Nation’s Educational, Scientific and Cultural Organization (UNESCO) Issues the Universal Declaration on Bioethics and Human Rights
The Declaration addresses ethical issues related to medicine, life sciences and associated technologies as applied to human beings, taking into account their social, legal and environmental dimensions. Although the Declaration is addressed to States, it also provides guidance to decisions or practices of individuals, groups, communities, institutions and corporations, public and private. The Declaration aims to:
(a) provide a universal framework of principles and procedures to guide States in the formulation of their legislation, policies or other instruments in the field of bioethics;
(b) guide the actions of individuals, groups, communities, institutions and corporations, public and private;
(c) promote respect for human dignity and protect human rights, by ensuring respect for the life of human beings, and fundamental freedoms, consistent with international human rights law;
(d) recognize the importance of freedom of scientific research and the benefits derived from scientific and technological developments, while stressing the need for such research and developments to occur within the framework of ethical principles set out in this Declaration and to respect human dignity, human rights and fundamental freedoms;
(e) foster multidisciplinary and pluralistic dialogue about bioethical issues between all stakeholders and within society as a whole;
(f) promote equitable access to medical, scientific and technological developments as well as the greatest possible flow and the rapid sharing of knowledge concerning those developments and the sharing of benefits, with particular attention to the needs of developing countries;
(g) safeguard and promote the interests of the present and future generations; and
(h) underline the importance of biodiversity and its conservation as a common concern of humankind.
Universal Declaration on Bioethics and Human Rights
2006
Ethical Theory and Methods
Publication of Bernard Gert, Charles Culver, and Danner Clouser’s Bioethics: A Systematic Approach
The authors state at the outset of their book, “We provide a systematic account of our common morality as a public system; we do not merely accept our thoughtful moral decisions and judgments but show how all of these decisions and judgments can be explained in terms of a common moral system. We then apply this common moral system to the moral problems that arise in the practice of medicine.” The authors argue that professionals in medical ethics lack an understanding of common morality or of its relation to the various branches of applied ethics and they propose to correct this.
B. Gert, C. Culver, and D. Clouser, Bioethics: A Systematic Approach (New York: Oxford University Press, 2006).
Bioethics: A Systematic Approach on Amazon
Social Justice
Publication of Madison Powers and Ruth Faden’s Social Justice: The Moral Foundations of Public Health and Health Policy
In their book, Powers and Faden bring our attention to the principle of justice in securing public health and designing health policy. They point out the nondistributional aspects of justice.
M. Powers and R. Faden, Social Justice: The Moral Foundations of Public Health and Health Policy (New York: Oxford University Press, 2006).
Social Justice: The Moral Foundations of Public Health and Health Policy
Narrative Ethics
Publication of Rita Charon’s Narrative Medicine: Honoring the Stories of Illness
Rita Charon and her colleagues originate the idea of narrative medicine as a form of clinical practice–”the narrative competence to recognize, absorb, interpret, and be moved by the stories of illness.” They argue that stories help us to understand why things happen. Charon’s work was crucial to the initial development of narrative ethics—the exploration of stories and storytelling and how they are connected to moral values.
R. Charon, Narrative Medicine: Honoring the Stories of Illness (New York: Oxford University Press, 2006).
Narrative Medicine: Honoring the Stories of Illness
Genomics and Ethics
The Cancer Genome Atlas (TCGA)
This cancer genomics program is founded by the National Cancer Institute and the National Human Genome Research Institute to molecularly characterize more than 20,000 primary cancers. The program is intended to improve the diagnosis, treatment, and prevention of cancer.
The Cancer Genome Atlas Program (TCGA)
Public Health Ethics
Approval of the Human Papilloma Virus Vaccine
This vaccine is first approved and may prevent up to 90% of cervical cancers. This first vaccine to protect women against cervical cancer received an expedited review through a priority review process.
Human Papillomavirus (HPV) Vaccines
Public Health Ethics
Publication of Sudhir Anand, Fabienne Peter, and Amartya Sen’s Public Health, Ethics, and Equity
This edited volume recognizes the importance of equity in public health ethics. It provides an interdisciplinary understanding of health equity and promotes the recognition of ethical issues in public health. It includes contributions from philosophers, anthropologists, economists, and public-health specialists on five major themes: defining health equity, health equity and social justice, responsibilities for health, and ethical issues in health evaluation.
S. Anand, F. Peter, and A. Sen, Public Health, Ethics, and Equity (Oxford: Oxford University Press, 2006).
Public Health, Ethics, and Equity
Reproductive Ethics
World Medical Association Declaration on Therapeutic Abortion (Declaration of Oslo)
The 57th World Medical Assembly (WMA) in October of 2006 declared that circumstances that bring the interests of a mother into conflict with the interests of her unborn child create a dilemma and raise the question as to whether or not the pregnancy should be terminated. The WMA recognized that diversity of responses is due in part to diversity of attitudes towards the life of the unborn child. The Association declared that individual conviction and conscience must be respected. It is not the role of the medical profession to determine the attitudes of any state or community but to attempt to ensure the protection of patients and the rights of physicians. Therefore, where the law allows therapeutic abortion to be performed, the procedure should be performed by a competent physician.
WMA Statement on Medically-Indicated Termination of Pregnancy
Narrative Ethics
Publication of Fitzhugh Mullan, Ellen Ficklen, and Kyna Rubin’s Narrative Matters: The Power of the Personal Essay in Health Policy
Publication of this book marks the beginning of the “Narrative Matters” essays in Health Affairs, and the similar essay format at The Journal of the American Medical Association’s “A Piece of My Mind.”
F. Mullan, E. Ficklen, and K. Rubin, Narrative Matters: The Power of the Personal Essay in Health Policy (Baltimore: Johns Hopkins University Press, 2006).
Narrative Matters: The Power of the Personal Essay in Health Policy on Amazon
2007
Ethics of Enhancement
Publication of John Harris’s Enhancing Evolution: The Ethical Case for Making Better People
In his 2007 book, John Harris endorses genetic engineering, stem-cell research, designer babies, and cloning. He argues that biotechnology makes human enhancement possible and in so doing biotechnology is good morally, for individuals, social policy, and good for future generations that need serious improvement. Biotechnology could allow us to live longer, healthier, and happier lives by, for example, providing us with immunity from cancer and HIV/AIDS. Biotechnologically driven enhancement could influence evolution to yield improved reasoning, concentration, and memory, strength, stamina, and reaction speed. He argues that it may not only be permissible but morally obligatory.
J. Harris, Enhancing Evolution: The Ethical Case for Making Better People (Princeton: Princeton University Press, 2007)
Enhancing Evolution: The Ethical Case for Making Better People
End-of-Life Issues
Medical Aid in Dying
Clinician-Patient Relationship
Seeking the Right to Commit Suicide While Healthy
A couple in British Columbia, Canada, Betty and George Coumbias, requested the legal right to commit suicide together, although only the husband was ill. They were the first couple to make such a request to complete simultaneous suicides with legal authorization. Ludwig Minelli, director of a Swiss assisted suicide group, Dignitas, petitioned the canton of Zurich to grant doctors authority to legally issue drugs for this purpose. They were featured in the documentary by John Zaritsky in 2007. The case was unusual in that Betty Coumbias was in excellent health.
‘Suicide Tourists’ Seek Place to Die
Arts and Ethics
Health Care Crisis of Uninsured Americans

Filmmaker Michael Moore makes a documentary portraying the American health care crisis and explores why millions of individuals in the U.S. lack health insurance coverage.
Sicko. 2007. Dog Eat Dog Films.
https://michaelmoore.com/movies/sicko/
2008
End-of-Life Issues
Rights of Parents to Make Medical Decisions for Children They Have Abused
The case focused on Andrew Bedner, a parent in Vermont charged with critically harming his child. If the child dies, the parent may be charged with murder. The court considered whether a parent who has abused a child may be legally allowed to make medical decisions. Jacob Appel, MD used the case to launch a drive to revise statutes governing parental decision-making in cases of abuse.
Mixed motives, mixed outcomes when accused parents won’t agree to withdraw care
Research Ethics
Publication of Jennifer S. Hawkins and Ezekiel J. Emanuel’s Exploitation and Developing Countries: The Ethics of Clinical Research
In this edited volume, philosophers and bioethicists reflect on the meaning of exploitation and consider whether it is inherently morally troubling. They examine whether and when clinical research in developing countries counts as exploitative and consider what can be done to minimize the possibility of exploitation in such circumstances.
J. S. Hawkins and E. J. Emanuel, Exploitation and Developing Countries: The Ethics of Clinical Research (Princeton: Princeton University Press, 2008).

Exploitation and Developing Countries: The Ethics of Clinical Research
Feminist Ethics
Journals in Bioethics
Publication of The International Journal of Feminist Approaches to Bioethics
This new journal publishes an article from Susan Sherwin entitled, “Whither Bioethics? How Feminism Can Help Reorient Bioethics,” in which she writes about feminism and bioethics.
S. Sherwin, “Whither Bioethics? How Feminism Can Help Reorient Bioethics,” International Journal of Feminist Approaches to Bioethics 1, no. 1 (2008): 8-27.
Whither Bioethics? How Feminism Can Help Reorient Bioethics
Reproductive Ethics
Religion and Ethics
Dignitas Personae
On September 8, 2008, a new doctrinal document was approved by Pope Benedict XVI to bring prior doctrines up to date by taking into account and evaluating new biomedical technologies. The doctrine asserted that the human embryo has from the beginning the dignity of a person. It disapproves of the use of artificial fertilization.
Instruction Dignitas Personae on Certain Bioethical Questions
2009
Research Ethics
Funding of Embryonic Stem Cell Research
The Obama administration announces it would expand National Institutes of Health funding for human embryonic stem research, which had been restricted under the Bush administration.
Obama Lifts Limit On Funding Stem Cell Research
Bioethics Commissions and Other Government Entities
Research Ethics Technology
Presidential Commission for the Study of Bioethics Issues
President Obama founded the Presidential Commission for the Study of Bioethical Issues in 2007. The Commission advised the President on bioethical issues arising from advances in biomedicine and related areas of science and technology. It produced over 65 educational materials until it ceased its activities in 2017.
https://bioethicsarchive.georgetown.edu/pcsbi/node/851.html
Social Justice
AMA Apology to Discrimination against African American Physicians
The American Medical Association apologizes for its history of discrimination against African American physicians on July 28, 2008. It apologized for excluding black physicians from membership in the AMA, for listing black doctors as “colored” in its physician directory, and for failing to speak against federal funding of segregated hospitals and in favor of civil rights legislation.
AMA Apology to African Americans
The history of African Americans and organized medicine
R. B. Baker et al., “African American Physicians and Organized Medicine, 1846-1968: Origins of a Racial Divide,” Journal of the American Medical Association 300, no. 3 (2008): 306-13.
African American Physicians and Organized Medicine, 1846-1968
Informed Consent
Publication of Frank Miller and Alan Wertheimer’s The Ethics of Consent: Theory and Practice
Frank Miller and Alan Wertheimer edit a volume exploring the important moral basis of consent in civil society. They explore the relationship of consent to autonomy and paternalism. They consider the role of consent in many contexts including sexual relations, employment, business, political arena, medical care, and clinical research.
F. Miller and A. Wertheimer, The Ethics of Consent: Theory and Practice (New York: Oxford University Press, 2008).
The Ethics of Consent: Theory & Practice
Organizational Ethics
IntegratedEthics®
The National Center for Ethics in Health Care at the Veterans Health Administration launches IntegratedEthics® Program. The program was designed to establish a national, standardized, comprehensive, systematic, integrated approach to ethics in health care. The national education and organizational change initiative is based on established criteria for performance excellence in health care organizations, methods of continuous quality improvement, and proven strategies for organizational change. The goal is to support, maintain, and improve ethics quality in health care.
E. Fox et al., “IntegratedEthics: An Innovative Program to Improve Ethics Quality in Health Care,” Innovation Journal 15, no. 2 (2010): 1-36.
IntegratedEthics: An Innovative Program to Improve Ethics Quality in Health Care
Ethics and Art
Precious

This fictional movie about a young African American woman who suffers from poverty and abuse wins wide acclaim and awards at several film festivals. It was based on the book, Push by Sapphire that vividly portrays the experience of sexual abuse and the possibility of escape. The movie provided a highly popular artistic expression of the ethically troubling aspects of sexual abuse and domestic violence and the difficulties that survivors face in overcoming these experiences.
Bioethics Organizations
The Australasian Association of Bioethics & Health Law (AABHL)
The AABHL is formed from the amalgamation of the Australasian Bioethics Association and the Australian and New Zealand Institute of Health Law and Ethics, both established in the early 1990s.
2010
Ethics of Enhancement
Publication of Alan Buchanan’s Beyond Humanity? The Ethics of Biomedical Enhancement
Alan Buchanan endorses efforts to enhance human life through biotechnology and refutes the arguments of those who consider enhancement a mistake.
A. Buchanan, Beyond Humanity? The Ethics of Biomedical Enhancement (Oxford: Oxford University Press, 2010).
Beyond Humanity? The Ethics of Biomedical Enhancement
Ethics of Health Policy
The Patient Protection and Affordable Care Act
The Patient Protection and Affordable Care Act was signed into law by President Obama on March 23, 2010. One of its principal aims was to expand insurance coverage to millions of uninsured Americans.
Patient Protection and Affordable Care Act, 42 U.S.C. § 18001 et seq. (2010).
Timeline of the Affordable Care Act
Ethics of Health Policy
Founding of the Patient-Centered Outcomes Research Institute (PCORI)
The U.S. Congress authorizes the creation of PCORI, an independent nonprofit nongovernmental organization to improve the quality and relevance of evidence to help patients, caregivers, and clinicians to make better-informed health care decisions. The creation of PCORI reflects a shift towards empowering patients by giving them a more active role in making decisions about their care and managing their own health.
Research Ethics
Responsible Conduct of Research Training
The National Science Foundation announces Responsible Conduct of Research (RCR) training requirements for funded investigators, students, and trainees. National Institutes of Health expands RCR training requirements.
Responsible and Ethical Conduct of Research
Research Ethics
Uncovering the Guatemala Sexually Transmitted Disease Studies
Susan Reverby, a professor at Wellesley College, finds documents concerning unethical research experiments conducted by the U.S. government in Guatemala from 1946-48. The experiments involved intentionally infecting over 1,300 subjects with venereal disease to assess the effectiveness of penicillin. Her detective work led to an apology by President Obama.
Research Ethics
Retraction of False Scientific Claims
The Lancet retracts a fraudulent paper published by Andrew Wakefield and colleagues in 1998 that had reported a link between autism and the measles vaccine. The publication highlights the importance of scientific integrity. Despite the retraction, public fear of vaccination has persisted.
Lancet retracts 12-year-old article linking autism to MMR vaccines
Research Ethics
Publication of Rebecca Skloot’s The Immortal Life of Henrietta Lacks
Journalist Rebecca Skloot, with the assistance of Deborah Lacks, tells the story of Henrietta Lacks who died of cervical cancer in 1951. Henrietta Lacks’s cancer cells became the first immortalized human cell line, HeLa cells, without the knowledge of her family.
R. Skloot, The Immortal Life of Henrietta Lacks (New York: Random House/Broadway Paperbacks, 2010).

The Immortal Life of Henrietta Lacks on Amazon
Ethical Theory and Methods
Publication of A.J. Simmons’s Article on Nonideal Theory
John Rawls introduced the distinction between ideal and nonideal theory in his influential book, A Theory of Justice. In this paper, Simmons clarifies and endorses Rawls’ distinction which, as he points out, is so useful in characterizing the relationship between philosophical theory and political practice.
A.J. Simmons, “Ideal and Nonideal Theory,” Philosophy and Public Affairs, 38, no. 1 (2010): 5-36.
Sexual and Gender Ethics
International Project on Bioethics, Sexuality, and Gender Identity
The project, founded by Lance Wahlert and Autumn Fiester at the University of Pennsylvania, unites over 300 bioethics scholars from across the globe.
Queer bioethics now an academic program at Penn
2011
Professional Ethics
Threat to Professional Speech
Florida passed a law barring doctors from discussing the dangers of gun ownership with patients. The law was overturned in 2017 in a ruling that indicated that the law violated doctors’ right to free speech.
https://media.ca11.uscourts.gov/opinions/pub/files/201214009.enbc.pdf
Informed Consent
Sexual and Gender Ethics
Nonconsensual Sex Reassignment
Christiane Völling, an adult intersex woman in Germany, is the first intersex person known to have successfully sued for damages in a case brought for nonconsensual surgical sex reassignment.
In re Völling, Regional Court Cologne, Germany
End-of-Life Issues
Clinician-Patient Relationship
The Royal Society of Canada International Expert Panel on End-of-Life Issues
The panel, chaired by Udo Schuklenk, publishes its report which had a significant impact on subsequent end-of-life legislation in Canada and in Australia. The report fostered a discussion about decriminalization of voluntary euthanasia and assisted suicide and facilitated legislation for this purpose.
Social Justice
Body and Soul
Sociologist Alondra Nelson publishes Body and Soul which reports on African American responses to medical discrimination in the United States and focuses on health activism by the Black Panther Party.
A. Nelson, Body and Soul (Minneapolis: University of Minnesota Press, 2011).
2012
Research Ethics
Possible Applicability of Research to Bioterrorism
Two papers, one by Ron Fouchier in the Netherlands and the other by Yoshihiro Kawaoka in Wisconsin are published in Science and Nature after debate about the possibility that the papers would facilitate the conduct of bioterrorism. The papers reported the results of NIH-sponsored research in which H1N1 avian flu virus was genetically modified so that it could be transmitted between mammals including humans.
One of Two Hotly Debated H5N1 Papers Finally Published
Resource Allocation
The Choosing Wisely Campaign
Prompted by Dr. Howard Brody’s New England Journal of Medicine publication in 2010, “Medicine’s Responsibility for Health Care Reform–The Top Five List,” and the National Physicians Alliance that piloted the “Five Things” concept, the American Board of Internal Medicine Foundation and Consumer Reports launches the Choosing Wisely Campaign, in which they seek to begin a national dialogue on avoiding unnecessary medical tests, treatments, and procedures.
Ethics and Art
How to Survive A Plague

This film documents how a group of young men and women, many of whom are HIV positive, formed two coalitions–ACT UP and TAG (Treatment Action Group)–the core of a movement that pushed for research to develop treatment of the AIDS infection.
https://surviveaplague.com/trailer
Sexual and Gender Ethics
First National Meetings
The first national symposium on “Queer Bioethics” (March 29, 2012) and the first national conference on “Bioethics, Sexuality, and Gender Identity: A National Conference” (September 21-22, 2012) are both held at the University of Pennsylvania.
Queer Bioethics Comes to Life at Penn
2013
Reproductive Ethics
End-of-Life Issues
Clinician-Patient Relationship
Controversy over Withdrawing Life Support from a Pregnant Woman
The husband and family of a woman in Texas, Marlise Munoz, who is declared brain-dead by her physician, wanted to remove her from life support. The hospital kept her on life support because it claimed that it could not legally withdraw life support from a pregnant patient. Texas law restricts the application of advance directives in pregnant patients, but the husband argued that the law was not applicable since his wife was brain dead. The judge ordered the hospital to remove organ support.
www.62daysmovie.com
End-of-Life Issues
Clinician-Patient Relationship
Controversy over Maintaining Ventilation after Declaration of Brain Death

Jahi McMath is declared brain-dead in California and her family wishes to maintain her body on mechanical ventilation.
NY Times: A Brain Is Dead, a Heart Beats On
McMath v. State of California U.S. N.D. Calif. No. 3:15-cv-06042; (Dismissal) U.S. N.D. Calif. No. 4:15-cv-06042-HSG
2014
Research Ethics
National Institutes of Health Addresses the Lack of Reproducibility of Scientific Evidence
The NIH launches the reproducibility initiative in response to problems with the reproducibility of scientific research.
Policy: NIH plans to enhance reproducibility
Enhancing Reproducibility through Rigor and Transparency
End-of-Life Issues
Clinician-Patient Relationship
Publication of Atul Gawande’s Being Mortal: Medicine and What Matters in the End
Atul Gawande writes in this book about the struggle in the medical establishment to forthrightly face the inevitability of death and how to reform practice to improve the experiences of patients as they approach the end of their life.
A. Gawande, Being Mortal: Medicine and What Matters in the End (New York: Metropolitan Book, Henry Holt and Company, 2014).
Arts and Ethics
Publication of Therese Jones, Delese Wear, and Lester Friedman’s Health and Humanities Reader
This is an extensive collection of essays by scholars, educators, artists, and clinicians that portrays the work that has emerged and offers fresh approaches to the health humanities literature.
T. Jones, D. Wear, and L. Friedman, Health and Humanities Reader (New Brunswick: Rutgers University Press, 2014).
Health Humanities Reader on Rutgers University Press
2015
Medical Aid in Dying
End-of-Life Issues
Clinician-Patient Relationship
Legality of Assistance in Dying in Canada
The Carter vs. Canada case opens Canada to medical assistance in dying (MAID) when the British Columbia Supreme Court strikes down the prohibition against assisted dying and allows a patient with amyotrophic lateral sclerosis to request assistance before she becomes too disabled to commit suicide on her own.
Carter v. Canada (AG), 2015 SCC 5
Legislative Background: Medical Assistance in Dying
Professionalism and Ethics
American Nurses Association (ANA) reissues Code of Ethics

The ANA declares 2015 the “Year of Ethics.” The organization aims to recognize the impact of ethical practice on patient safety and the quality of care. Earlier formulations of nursing ethics often took the form of Nightingale Pledges, a version of the Hippocratic Oath formulated for nurses introduced by a Detroit nursing school in 1893, revised in 1935, and often modified by individual nursing schools or institutions.
Reproductive Ethics
Birth of First Baby Following Mitochondria Replacement
A U.S.-based team working in Mexico helps Jordanian parents to have a child by a new 3-parent technique which involves mitochondrial DNA replacement in the mother’s ovum.
Exclusive: World’s first baby born with new “3 parent” technique
2016
Ethics of Health Policy
End-of-Life Issues
Clinician-Patient Relationship
Medicare Reimbursement for Conversations about End-of-Life Care
Medicare begins to reimburse physicians for conversations about preferences for end-of-life care. Long an issue of bipartisan agreement, the idea of reimbursing for discussions about end-of-life preferences had become a political lightening rod and was removed from the 2010 Affordable Care Act after it was mischaracterized as a “death panel” during the presidential election campaign and subsequent debates over the Act.
Doctors Ponder Delicate Talks As Medicare Pays For End-Of-Life Counsel
Research Ethics
National Institutes of Health (NIH) Moratorium on Creation of Human-Animal Chimeras
NIH issues a moratorium on research that creates human-animal chimeras. The NIH ban does not prevent scientists at the Salk Institute from creating the first pig-human chimera embryo in 2017.
NIH moves to lift moratorium on animal-human chimera research
Interspecies Chimerism with Mammalian Pluripotent Stem Cells
Ethics of Health Policy
American Medical Association (AMA) Revises Code of Medical Ethics
AMA issues a comprehensive revision to the Code of Medical Ethics for the first time since 1957. The AMA notes that the revisions are intended to improve “relevance, clarity and consistency,” primarily by bringing Opinions together and making the Code easier to navigate and search.
AMA publishes updated Code of Medical Ethics for contemporary medicine
Medical Aid in Dying
End-of-Life Issues
Clinician-Patient Relationship
Ethics of Health Policy
Canada Legalizes Euthanasia
Canadian parliament passes bill C-14, legalizing medical aid in dying, including euthanasia, in cases of mentally competent adults experiencing “enduring and intolerable suffering” and in cases where death is “reasonably foreseeable.” The quoted phrases will become issues for subsequent litigation.
Medical Aid in Dying
End-of-Life Issues
Ethics of Health Policy
Clinician-Patient Relationship
Colorado Legalizes Medical Aid in Dying
Colorado becomes the 6th U.S. state to legalize medically assisted dying, passing a ballot initiative. Colorado’s initiative is unique in that it precludes hospitals and health systems from punishing physicians from participating in the aid-in-dying process, which becomes a contentious issue for religiously-affiliated health systems.
Colorado Center for Health & Environmental Data: Medical Aid in Dying
Medical Aid in Dying
End-of-Life Issues
Ethics of Health Policy
Clinician-Patient Relationship
Belgium Allows Euthanasia of Minors
Belgium allows the first minor to undergo legal euthanasia in that country, following adoption of a revised law in 2014 that expanded access to euthanasia in Belgium to people of all ages.
BBC News: Belgium minor first to be granted euthanasia
BBC News: Belgium’s parliament votes through child euthanasia
Ethics of Health Policy
United Kingdom “Brexit” Vote
The United Kingdom narrowly votes to leave the European Union in the “Brexit” vote. Opponents worry that the vote puts at risk the >10% of doctors and 5% of nurses in the United Kingdom who are European Union migrants, as well as EU funding sources for medical and social science research on health care in the United Kingdom.
BBC News: Brexit: What you need to know about the UK leaving the EU
Research Ethics
Technology
Bioethics Commissions and Other Government Entities
U.S. National Academies Issues Report on Use of Gene Drives
National Academies of Sciences, Engineering, and Medicine publishes a report with recommendations for research on and use of gene drives, recognizing that this new use of genetic technologies could permanently alter wild populations of organisms. This is an early instance of a U.S. group endorsing the precautionary governance of an emerging technology.
Gene Drive Research in Non-Human Organisms: Recommendations for Responsible Conduct
Research Ethics
Technology
Ethics and Health Policy
Approval Allowing Embryos to Be Permanently Genetically Altered
The U.K.’s Human Fertilization and Embryology Authority authorizes the clinical use of two different techniques (maternal spindle transfer and pronuclear transfer) to prevent the transmission of mitochondrial DNA disorders, marking the first nationally-regulated approval process that allows embryos to be permanently genetically altered.
Techniques to prevent mitochondrial DNA disorders approved for use in clinics
2017
Informed Consent
Research Ethics
Charlie Gard Case
Charlie Gard was born in the United Kingdom in 2016 with an incurable and severe disease for which his parents eventually sought use of an experimental therapy. The U.K.’s National Health Service clinicians caring for him argued that experimental treatment would be futile and not in his best interest following seizures that produced severe brain injury. A series of court rulings in 2017 supported the NHS position as did, eventually, the U.S. researcher offering the experimental therapy. Charlie died in July 2017, the day after his mechanical ventilation was stopped. The case was widely, though erroneously, tied to monetary concerns (the NHS initially agreed to pay for the therapy, and when they argued against it, his parents easily raised the funds to pay for the experimental therapy using a GoFundMe page) and hence to U.S. health policy arguments. U.S. Vice President Mike Pence, for example, said of the case at the time, “ . . . for all the talk on the left about single-payer, that’s where it takes us . . .” (i.e., asserting single payer health coverage leads to government rationing).
Ethics, conflict and medical treatment for children: From disagreement to dissensus
Ethics and Health Policy
Professionalism
Florida Law Banning Physicians from Speaking to Patient about Guns Overturned
An appeals court overturns a Florida law that would have banned physicians from speaking with patients about guns in the home, ruling that it violated doctors’ right to free speech.
Court Strikes Down Florida Law Barring Doctors From Discussing Guns With Patients
Research Ethics
Ethics of Health Policy
Removal of Science Web Pages by the Incoming Trump Administration
On the day of the 45th presidential inauguration, web pages on health care, civil liberties, climate change, and sexual and gender ethics issues are abruptly removed from the White House web site. And shortly after President Trump takes office, thousands of web pages from science-based federal agencies are taken down and scientists at the Environmental Protection Agency and Department of Agriculture are blocked from communicating with the public and the press about their research. These steps are part of a set of policy initiatives that are widely interpreted as an assault on science by the new administration.
Scientific American: Climate Web Pages Erased and Obscured under Trump/
Scientific American: Trump Administration Restricts News from Federal Scientists at USDA/EPA
Ethics of Health Policy
Research Ethics
First March for Science
The inaugural March for Science is held in Washington, D.C. and multiple other sites around the country in response to threats to science funding, transparency, and integrity. Total participation is estimated at over one million people worldwide, with more than 100,000 in Washington, D.C. This has since become an annual event, held on Earth Day each year. Robert Proctor, a historian of science at Stanford University, stated that the initial March for Science was “pretty unprecedented in terms of the scale and breadth of the scientific community that’s involved” and was rooted in “a broader perception of a massive attack on sacred notions of truth that are sacred to the scientific community.”
Historians say the March for Science is ‘pretty unprecedented’
Medical Aid in Dying
End-of-Life Issues
Ethics of Health Policy
Clinician-Patient Relationship
Washington, D.C. Legalizes Assisted Dying
Washington, D.C. becomes the 7th U.S. jurisdiction to allow legal medical aid in dying.
D.C. physician-assisted suicide law goes into effect
Medical Aid in Dying
End-of-Life Issues
Ethics of Health Policy
Clinician-Patient Relationship
Victoria, Australia Legalizes Assisted Dying
Victoria becomes the first Australian state to allow medical aid in dying.
NY Times: Euthanasia Law Passes in Australia for First Time
Ethics of health policy
End-of-Life Issues
Clinician-Patient Relationship
The U.S. Department of Veterans Affairs Creates National System for Cataloguing End-of-Life Preferences among Veterans
The U.S. Department of Veterans Affairs implements the “Life Sustaining Treatment Decisions Initiative,” creating a national system for eliciting goals and cataloguing end-of-life preferences.
https://www.ethics.va.gov/LSTDI.asp
2018
Research Ethics
Revised Common Rule
Seventeen federal agencies had published final revisions to the “Common Rule” governing human subjects research in 2017, but these eventually come to be called the “2018 Common Rule Requirements,” and actual implementation is delayed until 2019. The rules specifically exempt some activities from consideration as human subjects research (e.g., public health surveillance and activities like oral histories, journalistic and biographical investigations, and historical scholarship) and streamline IRB review processes, among other changes.
US Department of Health & Human Services Revised Common Rule
Ethics of Health Policy
Professionalism
End-of-Life Issues
Clinician-Patient Relationship
Creation of the Human Health Services (HHS) Conscience and Religious Freedom Division
The Human Health Services announces the formation of a new “Conscience and Religious Freedom Division” of its Office of Civil Rights, which aims to protect health professionals who refuse to deliver certain health care services due to personal or religious conscience objections (e.g., abortions).
US Department of Health & Human Services: Conscience & Religious Freedom
Research Ethics
Technology
The First Genome-Edited Babies Are Born

The first two genome-edited babies are born in China. Defying international agreements and laws in his home country, the Chinese biophysicist He Jiankui used a CRISPR gene-editing tool to modify the genes of two embryos in an apparent attempt to make them resistant to HIV infection. His work was roundly condemned both within and outside China, but it was soon followed by an announcement from Russia that a scientist there also planned to create genome-edited babies, raising questions about whether and how the international community might reign in rogue scientists.
AP News: Chinese researcher claims first gene-edited babies
End-of-Life Issues
Technology
Ethics of Health Policy
Clinician-Patient Relationship
Publication of Defining Death: Organ Transplantation and the Fifty-Year Legacy of the Harvard Report on Brain Death
The Hastings Center publishes this special report on the definition of death.
Defining Death: Organ Transplantation and the Fifty-ear Legacy of the Harvard Report on Brain Death
Hastings Center Report: What Does “Dead” Mean?
Ethics of Health Policy
Research Ethics
Federal “Right to Try” Law Passed
President Trump signs a federal “right-to-try” bill allowing patients to petition companies to use experimental drugs without oversight by the U.S. Food & Drug Administration. The bill does not require that companies provide drugs for use and very few people receive drugs under its auspices in the first two years after its passage.
US FDA: Federal “Right to Try” Law
Professionalism
Ethics Committees and Ethics Consultation
Ethics Consultant Certification Program Implemented
The American Society for Bioethics and Humanities implements the Healthcare Ethics Consultant Certification Program and the inaugural group of certified consultants is named.
American Society for Bioethics & Humanities: Healthcare Ethics Consultant-Certified Program
Medical Aid in Dying
End-of-Life Issues
Ethics of Health Policy
Clinician-Patient Relationship
Canadian Reports on Controversies in Assisted Dying
Canadian parliament receives three expert panel reports on medical aid in dying from the Council of Canadian Academies, exploring the possibility of extending medical assistance in dying to mature minors, people with psychiatric conditions, and those making requests in advance. These reports followed on the highly-publicized case of Audrey Parker, a Nova Scotia woman who chose to end her life earlier than she would have wanted for fear that she might lose mental capacity if she waited.
https://Council of Canadian Academies Medical Assistance in Dying
CBC: Halifax woman posthumously calls for fix to Canada’s assisted dying rules
Medical Aid in Dying
End-of-Life Issues
Ethics of Health Policy
Clinician-Patient Relationship
Hawaii Legalizes Assisted Dying
Hawaii becomes the 8th U.S. jurisdiction to allow medical aid in dying.
Ethics of Health Policy
Professionalism
The “Stay in Their Lane” Social Media Firestorm
After the American College of Physicians publishes a policy paper on preventing gun violence, the National Rifle Association sparks a social media firestorm by telling “self-important anti-gun doctors to stay in their lane” on Twitter.
Forbes: How Doctors Responded After NRA Told Them To ‘Stay In Their Lane’
2019
Dan Callahan, Cofounder of The Hastings Center, Dies

A prodigious author and one of the world’s preeminent bioethics scholars, Dan Callahan cofounded The Hastings Center, the world’s first bioethics research institute, with Willard Gaylin in 1969.
Sexual and Gender Ethics
Caster Semenya Ruled Ineligible to Compete as a Woman

Caster Semenya, a female South African middle distance runner is declared ineligible for any future International Association of Athletics Federations women’s events unless she takes medication to suppress her testosterone levels. This followed on years of debate about whether Semenya and others with “differences of sex development” should be allowed to compete as women despite having testosterone levels comparable to men.
NY Times: Caster Semenya Barred From 800 Meters at World Championships
Medical Aid in Dying
End-of-Life Issues
Ethics of Health Policy
Clinician-Patient Relationship
New Jersey Legalizes Assisted Dying
New Jersey becomes the 9th U.S. jurisdiction to allow medical aid in dying.
CNN: New Jersey will now allow terminally ill patients to end their lives
Medical Aid in Dying
End-of-Life Issues
Ethics of Health Policy
Clinician-Patient Relationship
Maine Legalizes Assisted Dying
Maine becomes the 10th U.S. jurisdiction to allow medical aid in dying.
AP: Maine becomes 8th state to legalize assisted suicide
Public Health Ethics
Novel Coronavirus (SARS-CoV-2) Outbreak in Wuhan, China
The outbreak of the 2019 coronavirus disease (COVID-19) was first reported on December 31, 2019 in Wuhan China.
2020
Public Health Ethics
Ethics of Health Policy
Coronavirus (SARS-CoV-2) Pandemic
Massive outbreaks of Covid-19 (the illness caused by SARS-CoV-2) arise initially in China, rapidly becoming a pandemic, overwhelming health systems in Italy, Iran, Spain, and Brazil, as well as New York, California, Florida, Texas, Arizona, and many other countries, states, and cities. Resource shortages prompt development and implementation of plans for contingency and crisis standards of care. The latter were rarely implemented, despite apparent need, which forced consideration of public health ethics and ethically justifiable strategies to allocate scarce resources, such as ICU beds and mechanical ventilators, often focusing on the role of equity. Debates also arise around the relative value and costs of nonpharmaceutical interventions, especially “social distancing” measures such as masking, quarantine, and economic shutdowns. Extreme examples of racial, ethnic, and socioeconomic disparities in Covid-19 infection rates and outcomes are also reported. Though these disparities were not surprising given longstanding, well-documented underlying disparities in health care, they draw particular attention as they are reported at almost the same time as the emergence of widespread protests over racial injustice in policing, sparked by the murder of George Floyd (see below).
WHO: Naming the coronavirus disease (COVID-19) and the virus that causes it
Social Justice
Black Lives Matter Movement (George Floyd protests)

Created in 2013 in response to the death of Trayvon Martin and subsequent acquittal of his killer, the Black Lives Matter movement in 2020 becomes a massive “international response to state-sanctioned violence and anti-Black racism,” galvanizing around the videotaped, brutal killing of an unarmed black man named George Floyd by police officers in Minneapolis. Protests over police killings sweep many U.S. cities and the focus quickly expands to encompass racial injustice (and anti-Black racism in particular) in health care and other areas where the legacy of “scientific racism” perpetuates white privilege and structural discrimination again Black and brown people.
Social Justice
American Academy of Pediatrics Apologizes for Its Racist Past
Joining the American Medical Association, where the AMA Institute for Ethics had carried out an investigation into racist membership policies prompting the organization to issue a public apology in 2008, the American Academy of Pediatrics in 2020 concluded an investigation into its prior racist membership policies and issued an apology, seeking a “path to equity.”
Truth, Reconciliation, and Transformation: Continuing on the Path to Equity
2022
Willard Gaylin, Cofounder of The Hastings Center, Dies

Willard Gaylin, an acclaimed psychiatrist and psychoanalyst and a pioneering scholar in bioethics who cofounded The Hastings Center, died on December 30. He was 97. Gaylin founded The Hastings Center (originally called the Institute of Society, Ethics and the Life Sciences) in 1969 with Daniel Callahan.
REPRODUCTIVE ETHICS
Federal Right to Abortion Is Eliminated by the United States Supreme Court (Dobbs v. Jackson Women’s Health Organization, 597 U.S. 215)
This landmark decision of the U.S. Supreme Court eliminated the constitutional right of a pregnant woman to choose to have an abortion, established by Roe v. Wade in 1973 and upheld by Planned Parenthood of Southeastern Pennsylvania v. Casey in 1992. The Dobbs decision was released on June 24, 2022, giving states the authority to regulate and ban abortion.
Hastings Center Bioethics Timeline Appendix
Bioethics timeline photo credits
Timeline designed by Nora Porter and Julie Chibbaro
Click the terms below to navigate the Timeline by topic, or use “command f” on your keyboard to search for any word or phrase on the page.


